
L) after the Eruption of Mount Sinabung using Atomic Absorption Spectrophotometer

Analysis of Arsenic in Purple Cabbage (Brassica Oleracea var.
capitata L) after the Eruption of Mount Sinabung using Atomic
Absorption Spectrophotometer
Boby Cahyady
1
, Suharman
1
, Muhammad Taufik
1
, Zul Alfian
1
, Mariany Razali
2
and Desi Ardilla
3
1
Chemistry Department, Universitas Sumatera Utara, Medan 20155, Sumatera Utara, Indonesia
2
Pharmacy Department, Universitas Tjut Nyak Dhien, Medan, Indonesia
3
Agricultural Technology Department, Universitas Muhammadiyah Sumatera Utara, Medan, Indonesia
Keywords: Arsenic, Cabbage, Analysis, Negative Impact, Atomic Absorption Spectrophotometer.
Abstract: The negative impact after the eruption of Mount Sinabung is the exposure of heavy metals to plants and
animals around the mountain. The heavy metal commonly contained in vegetables is Arsenic. Many of the
people around Mount Sinabung work as cabbage farmers. Cabbage plants that have many benefits,
especially in medicine are purple cabbage (Brassica oleracea var. Capitata L). This work aims to analyze
the levels of arsenic contained in purple cabbage after the eruption of Mount Sinabung. The sampling
technique uses simple random sampling. Sampling was carried out at 5 points of collection which is 50 m
from Mount Sinabung. In this work, Dry destruction method was developed using HCl and nitric acid.
Instrument Atomic Absorption Spectrophotometers (AAS) equipped with Vapor Hydride Generation
Acessories were developed to analyze Arsenic levels. At a wavelength of 193.7 nm, The concentration of
arsenic in purple cabbage was obtained at the sampling points 1, 2, 3, 4, and 5 respectively: 0.4755, 0.5808,
0.6534, 0.5517, 0, 5481 mg / Kg. This result is lower than the maximum limit of arsenic contamination in
vegetables, which is 1.0 mg / Kg. (SNI No. 7387: 2009).
1 INTRODUCTION
Mount Sinabung is located on the Karo Plateau in
Karo Regency, North Sumatra. The mount Sinabung
and Mount Sibayak are two active volcanoes in
North Sumatra (Lee, Lu, and Kim 2017). Mount
Sinabung is 2,460 meters above sea level. The
mountain began to erupt again on August 29, 2010.
In early 2019, Mount Sinabung began to erupt and
emitted hot lava. (Kusmartini et al. 2017).
In this case, the various activities of Mount
Sinabung have both positive and negative impacts
on local residents who will immediately feel the
negative impact, for example when Mount Sinabung
erupts, Mount Sinabong emits hot clouds and lava
that carry a lot of energy when it flows. Faded white
volcanic dust covers the surrounding forests, villages
and agricultural land (Nain Felix Sinuhaji 2011), so
it is necessary to conduct studies on the dangers of
volcanic ash for local residents, plant health, the
condition of local livestock, and the dangers posed
to crops and livestock (Lee, Lu, and Kim 2017). The
volcanic dust after the eruption of Mount Sinabung
produces heavy metals such as arsenic and various
other heavy metals which have an impact on the
quality of agricultural products including cabbage
(Harahap 2019)
Arsenic is the most toxic chemical and
metalloid in nature, and is also an important element
that needs attention, because even at low
concentrations, arsenic can cause toxicity and
carcinogenicity (Golui et al. 2017). Arsenic
exposure to humans can be inorganic or organic.
Arsenic in the environment can be in the form of
natural substances or pollution caused by human
activities (Šlejkovec et al. 2021). Arsenic can be
found in water, air, food and soil, including volcanic
eruptions, mining pollution, and use of pesticides
and fertilizers (Hazimah and Triwuri 2018). The
toxic effects of arsenic are well known, but depend
on the organic or inorganic form of the arsenic
compound (Harahap 2019).
Inorganic arsenic compounds are more toxic
than organic compounds (Hazimah and Triwuri
2018). Arsenic is a carcinogen because long-term
exposure increases the risk of many types of cancer,
Cahyady, B., Suharman, ., Taufik, M., Alfian, Z., Razali, M. and Ardilla, D.
Analysis of Arsenic in Purple cabbage (Brassica oleracea var. Capitata.
DOI: 10.5220/0010614200002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 592-595
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
593

including skin cancer, bladder cancer, lung cancer,
kidney cancer, liver cancer, and prostate cancer
(Rokayya et al. 2013). The effects of arsenic are
related to changes in the gastrointestinal tract,
cardiovascular system, blood, lungs, nerves,
immunity, reproduction and long-term effects
caused by arsenic (Fikri, Setiani, and Nurjazuli
2012). According to a study by the International
Agency for Research on Cancer (IARC), arsenic is
listed as a category first carcinogen, suggesting that
arsenic can cause human lung cancer, skin cancer
and bladder cancer. There is no minimum threshold,
and small amounts of arsenic can be harmful to the
human body for human health (Singh, Kumar, and
Sahu 2007).
Ginting, E.E. (2018) was reported about arsenic
(As) in rice. Rice circulating in the community still
contains arsenic. The highest arsenic content is
found in rice circulating in Medan of 3.40 mg / kg
(brown rice), 0.33 mg / kg (white rice), and 0.13 mg
/ kg (black rice) (Ginting, Silalahi, and Putra 2018).
Ridwan, M.H. (2017) was studied about arsenic (As)
in spinach using AAS. The results showed that
arsenic levels = 0.35 mg / kg (green spinach) and
0.40 mg / kg (red spinach) (Harahap 2019). The
compound arsenic (As) was believed to have been
exposed to farmers' cabbage, which was exposed by
the eruption of Mount Sinabung. Analysis of
Arsenic Metal using AAS has also been studied in
University of Syiah Kuala Banda Aceh (Nasir,
Sulastri, and Hilda 2018). In this study, a dry
digestion method was developed on soil samples.
The AAS used is a hydride vapor generator. On
garden soil, arsenic was detected at 1.6860 ppm. In
this work, purple cabbage leaves were analyzed
using atomic absorption spectrophotometry (AAS)
by taking samples at 5 points.
2 METHOD
2.1 Material
The materials used include purple cabbage from the
post-eruption area of Sinabung Mount. Nitric acid
65% (Sigma Aldrich), Standard of Arsenic
(Germany), hydrochloric acid 37% (Merck), and
sodium hydroxide 97% (Sigma Aldrich).
2.2 Collecting Samples
The vegetables used as samples were purple cabbage
(Brassica oleracea var. Capitata L) which was in
the area after the eruption of Mount Sinabung.
Simple random sampling technique was applied in
this work. In this method, sample members are
selected directly from the entire population by not
dividing the population according to groups because
they are considered to have the same chance of
being selected. So in this way the population was
considered as one large group, where the sample was
taken to represent the population.
2.3 Destruction Process
Each sample taken from 5 points was weighed 50 g
and then dried in an oven at 100°C for 3 hours. The
samples were then placed in an oven at 400°C for 4
hours. Once in cold conditions, the sample was
dissolved with a mixture of 65% HNO
3
and 37%
HCl in a ratio of 5: 2. The next process is to heat it
on a hot plate until the sample dissolves. The sample
solution was transferred to a 100 ml volumetric
flask, then add demineralized water to the mark. The
resulting solution was analyzed using an atomic
absorption spectrophotometer (AAS) and equipped
with Vapor Hydride Generation Accessories.at a
wavelength of 193.7 nm.
2.4 Calibration Curves
Arsenic standard solution with a concentration of
1000 μg / ml was used as much as 10 mL and put in
a bottle 100 mL and sufficient with demineralized
water; up to the mark. In this process Arsenic is
produced with a concentration of 100 ppm. 5 mL of
the solution is pipetteed and put into a 500 mL
volumetric flask and then sufficient to mark the line
with aquademineral (concentration 1 μg / ml). The
solution with a concentration of 1 μg / ml was then
diluted into a standard solution with variations of 0.0
0.2 0.4 0.6 and 0.8 μg / ml.
2.5 Arsenic Analysis
The absorbance was measured using an atomic
absorption spectrophotometer at a wavelength of
193.7 nm. The absorbance and concentration values
will be plotted to obtain a calibration curve then the
regression equation is calculated, namely y = ax + b.
3 RESULT
3.1 Destruction Process
Determination of Arsenic (As) in Purple Cabbage
(Brassica oleracea var. Capitata L) taken from
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
594
Berastagi Sub district, Dry destruction was developed
in the furnace at 400° for 3 hours until white ash was
formed, then dissolved using HNO
3
and washed used
demineralized water, the filtering process was carried
out with Whatmann filter paper No 42.
In this work, HNO
3
functions to break down the
sample into compounds that are easier to decompose
while demineralized water was used to wash the
sample solution left on the filter paper. The dry
destruction method was used to break the bonds
between the organic compounds and the metal being
analyzed. Nitric acid was an oxidizing agent which
can decompose the sample into its elements.
3.2 Analysis of Arsenic Used AAS
The calibration curve of the arsenic (As) standard
solution was carried out by preparing a standard
series solution with various concentrations at 0.0 0.2
0.4 0.6 and 0.8 μg / ml. The absorbance value was
obtained using Atomic Absorption
Spectrophotometry (AAS). The conditions of the
Atomic Absorption Spectrophotometry (AAS)
instrument on the measurement of Arsenic
concentration can be seen in Table 3.1 and The
absorbance data of the Arsenic standard curve can
be seen in Figure 3.1 below.
Table 3.1: The conditions of the Atomic Absorption
Spectrophotometry (AAS) instrument.
No Parameter Condition
1 Wavelength (nm) 193,7
2 Flash type air- C
2
H
2
3 Burner Gas Flow Rate 2,0
4 height 7,5
5 Type of Lamp BGC-02
6 Gap Width (nm) 1,3
Table 3.1. shows that the As analysis uses the
AAS nm instrument with a hydride vapor generator
at a wavelength of 193.7 nm. The flash type is air-
C
2
H
2
, Burner Gas Flow Rate at 2.0, height = 7.5,
type of lamp BGC-02 and Gap Width (nm) = 1.3.
This condition was chosen because it is the optimum
condition for measuring or Arsen in a vegetable
sample.
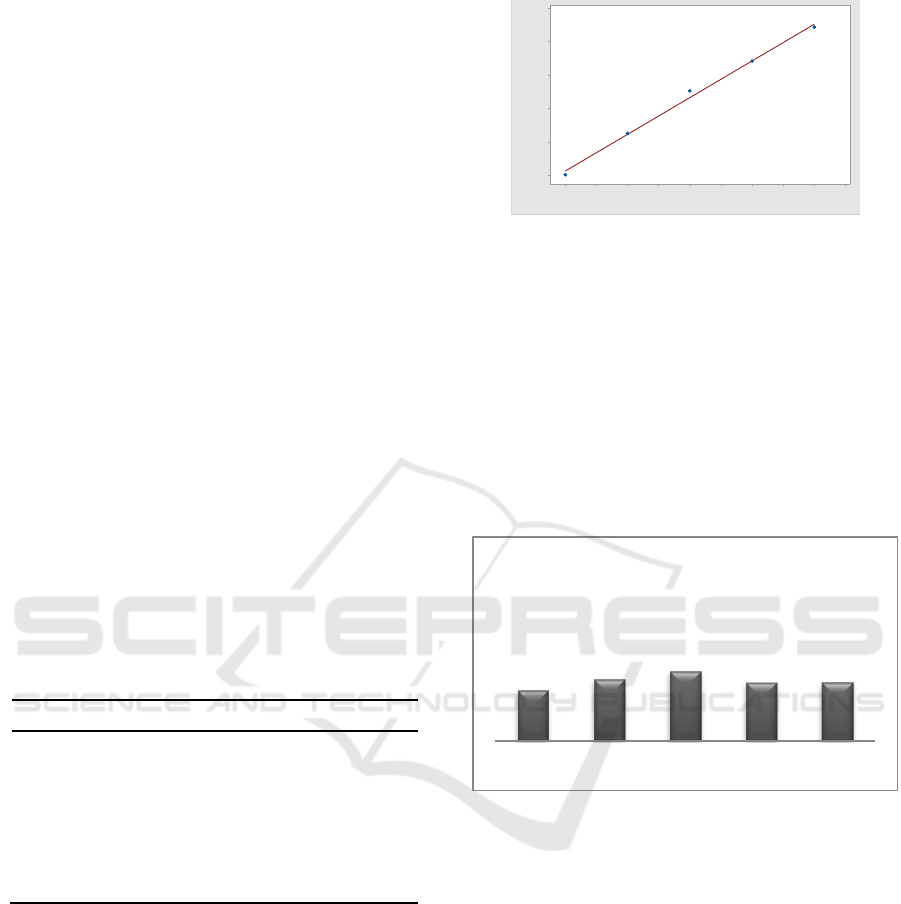
Figure 3.1: Arsenic standard curve.
Figure 3.1. shows the standard serial curve used
on AAS Instruments. The straight-line equation
obtained is y = 0.0551x + 0.0012. The resulting
correlation coefficient is 0.9947. Based on this data,
it can be seen that there is a positive correlation
between the concentration of the standard series
sample and the resulting absorbance value. Based on
the results of the calculations made, the levels of
Arsenic in purple Cabbage (Brassica oleracea var.
Capitata L) were obtained for points 1, 2, 3, 4, and 5
respectively as shown in Figure 3.2. the following :
Figure 3.2: The concentration of Arsenic in Purple
Cabbage (Brassica oleracea var. Capitata L).
Figure 3.2. showed that the levels of Arsenic
obtained in purple cabbage (Brassica oleracea var.
Capitata L) taken at 5 sample points were 0.4755,
0.5808, 0.6534, 0.5517, and 0.5481 mg / Kg
respectively. The results obtained are still below the
threshold, where the maximum value of Arsenic in
vegetables is 1 mg / Kg SNI No. 7387: 2009.
Arsenic is a heavy metal and can cause poisoning
if it accumulates in the human body. Heavy metals
have been detected in many vegetables. Especially
plants grown near highways and air pollution areas,
including plants from factory fumes and motor vehicle
fumes (Šlejkovec et al. 2021). Arsenic accumulates
mainly through plant organs (such as leaves, stems,
roots, and tubers), and accents can also accumulate
through food contaminated with heavy metals. If this
0,90,80,70,60,50,40,30,20,10,0
0,05
0,04
0,03
0,02
0,01
0,00
Concentration of As Standard (μg/ml)
Absorbantion (A)
0,4755
0,5808
0,6534
0,5517
0,5481
Sampel1Sampel2Sampel3Sampel4Sampel5
ConcentrationofAsinPurple
Cabbage
y = 0,0551x + 0,0012
R
²=
0,9947
Analysis of Arsenic in Purple cabbage (Brassica oleracea var. Capitata
595

situation continues for a long time, it can reach levels
that are harmful to human health. Arsenic in the body
can damage health in various ways, namely reducing
the number of red blood cells, decreasing hemoglobin
synthesis which causes anemia. Anemia occurs due to
the binding of arsenic with enzymes (Fikri, Setiani,
and Nurjazuli 2012). This results in inhibition of red
blood synthesis.
The mechanism of entry of Arsenic into the human
body can be through the oral respiratory system, or
directly through the skin surface. As much as 95% of
the arsenic is absorbed in the body, it is bound by
erythrocytes and then removed by the blood to the
body organs and then stored in soft tissues (bone
marrow, nervous system, kidneys, liver) and hard
tissues (bones, nails, hair, teeth) (Nasir, Sulastri, and
Hilda 2018) (Fikri, Setiani, and Nurjazuli 2012).
4 CONCLUSION
This research has analyzed the Arsenic metal in the
purple cabbage (Brassica oleracea var. Capitata L)
after the eruption of Mount Sinabung. The sampling
technique uses simple random sampling at 5 points
of collection which is 50 m from Mount Sinabung.
Dry destruction method was developed using
Atomic Absorption Spectrophotometers (AAS)
equipped with Vapor Hydride Generation Acessories
at 193.7 nm. The concentration of arsenic in purple
cabbage was obtained at the sampling points 1, 2, 3,
4, and 5 respectively: 0.4755, 0.5808, 0.6534,
0.5517, 0, 5481 mg / Kg. This result is lower than
the maximum limit of arsenic contamination in
vegetables, which is 1.0 mg / Kg. (SNI No. 7387:
2009)
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Rector of
University of Sumatera Utara for the financial
support via Penelitian Dosen Muda Tahun 2019
Contract No. : 318/UN5.2.3.1/PPM/KP-TALENTA
USU/2019 Date 2 April 2019.
REFERENCES
Fikri, Elanda, Onny Setiani, and Nurjazuli. 2012.
“Hubungan Paparan Pestisida Dengan Kandungan
Arsen ( As ) Dalam Urin Dan Kejadian Anemia (
Studi : Pada Petani Penyemprot Pestisida Di
Kabupaten Brebes ).” Jurnal Kesehatan Lingkungan
Indonesia 11 (1): 29–37.
Ginting, Evi Ekayanti, Jansen Silalahi, and Effendy Delux
Putra. 2018. “Analysis of Arsenic in Rice in Medan,
North Sumatera Indonesia by Atomic Absorption
Spectrophotometer.” Oriental Journal of Chemistry 34
(5): 2651–55. https://doi.org/10.13005/ojc/340557.
Golui, Debasis, D. N. Guha Mazumder, S. K. Sanyal, S. P.
Datta, P. Ray, P. K. Patra, S. Sarkar, and K.
Bhattacharya. 2017. “Safe Limit of Arsenic in Soil in
Relation to Dietary Exposure of Arsenicosis Patients
from Malda District, West Bengal- A Case Study.”
Ecotoxicology and Environmental Safety 144
(November): 227–35.
https://doi.org/10.1016/j.ecoenv.2017.06.027.
Harahap, Muhammad Ridwan. 2019. “Analisis Logam
Arsenik (As) Dan Kadmium (Cd) Pada Sayur Bayam
Hijau (Amaranthus Tricolor) Terhadap Bayam Merah
(Blitum Rubrum) Dengan Metode Spektrofotometri
Serapan Atom (SSA).” Aricis 1 1 (Cd): 500–505.
Hazimah, and Nurlinda Ayu Triwuri. 2018. “Analisis
Kandungan Arsenik (As) Dan Cianida (CN) Depot Air
Minum Isi Ulang Di Kota Batam.” Jurnal Rekayasi
Sistem Industri 3 (2): 129–33.
Kusmartini, I, W Y N Syahfitri, S Kurniawati, D D
Lestiani, and M Santoso. 2017. “Elemental
Characterization of Mt . Sinabung Volcanic Ash,
Indonesia by Neutron Activation Analysis.” Journal of
Physics: Conference Series 860 (1): 1–10.
Lee, Chang-wook, Zhong Lu, and Jin Woo Kim. 2017.
“Monitoring Mount Sinabung in Indonesia Using
Multi-Temporal InSAR.” Korean Journal of Remote
Sensing 33 (February 2018): 37–46.
https://doi.org/10.7780/kjrs.2017.33.1.4.
Nain Felix Sinuhaji. 2011. “Analisis Logam Berat Dan
Unsur Hara Debu Kabupaten Karo Vulkanik Gunung
Sinabung.”
Nasir, M, Sulastri, and Michelia Mutiara Hilda. 2018.
“Analisis Kadar Logam Timbal Dan Arsenik Dalam
Tanah Dengan Spektrometri Serapan Atom.” Jurnal
IPA Dan Pembelajaran IPA 02 (02): 89–99.
Rokayya, Sami, Chun Juan Li, Yan Zhao, Ying Li, and
Chang Hao Sun. 2013. “Cabbage (Brassica Oleracea
L. Var. Capitata) Phytochemicals with Antioxidant
and Anti-Inflammatory Potential.” Asian Pacific
Journal of Cancer Prevention 14 (11): 6657–62.
https://doi.org/10.7314/APJCP.2013.14.11.6657.
Singh, Nrashant, Deepak Kumar, and Anand P. Sahu.
2007. “Arsenic in the Environment: Effects on Human
Health and Possible Prevention.” Journal of
Environmental Biology 28 (2 SUPPL.): 359–65.
Šlejkovec, Zdenka, Leon Gorše, Ana Grobler, Marta
Jagodic, and Ingrid Falnoga. 2021. “Arsenic
Speciation and Elemental Composition of Rice
Samples from the Slovenian Market.” Food Chemistry
342 (December): 128348.
https://doi.org/10.1016/j.foodchem.2020.128348.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
596
