
Optical Study of Graphene Quantum Dots from
Sawahlunto Coal Graphite
Dellyansyah
1,2
, Saharman Gea
2,3
, Andriayani
2,3
, Mahyuni Harahap
2,3
and Grace Nainggolan
1,2
1
Postgraduate Chemistry Program, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Medan, Indonesia
2
Cellulosic and Functional Material Research Center, Universitas Sumatera Utara, Medan, Indonesia
3
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Medan, Indonesia
Keywords: Graphene quantum dots, Sawahlunto coal graphite (BB900), optical properties.
Abstract: Graphene quantum dots (GQDs), quantum sized graphene materials, have been proposed to be candidate in
optical and energy storage devices applications due its transparant properties and high surface area. In this
work, Sawahlunto coal graphite was converted into GQDs and analysed the optical properties. The coals
were dispersed in strong acid and irradiated with ultrasound before oxidized and hydrothermalized into
GQDs. UV-vis spectroscopy and photoluminescence spectrophotometer were used to determined the
conjugate structure and emission type respectively. The UV-vis spectra showed that the product had
conjugated structure in 230 nm. Photoluminescence results confirmed that the GQDs had cyan emission.
1 INTRODUCTION
Graphene quantum dots (GQDs), one of carbon
nanomaterials always takes an advanced part in
development of modern science and technology.
This quantum size exhibits excelent progress in
optoelectronic and energy storage device (Li et al.
2015). This is due to its transparant property and
high surface area (Yan and Liu, 2014).
GQDs can be obtained from graphene, graphene
oxide (GO), carbon nanotubes (CNTs), carbon fibers
and graphite as precursor. GO is be converted to be
GQDS by Hummers method and followed by redox
treatment repeatedly. This method is simple and
efective mass production, but requires high cost
(Bak, Kim and Lee, 2016). Hence, several literatures
have reported an advance investigation of GQDs
with low cost and high yield such as using high
temperature autoclave (Sun et al. 2013) and
microwave treatment (Shin et al., 2014).
Coal, a high carbon compound material, is able
to produce graphene to subtitute graphite (Powell
and Beall, 2015). Coal from Sawahlunto, West
Sumatera is a kind of a high volatile bituminous
with 40,79% - 49,67 % of carbon. It is potentially
converted into GQDs. It had been reported that coal
from Sawahlunto can be converted into graphene by
exfoliation graphitization graphite at 900
o
C
(Purwandari et al. 2020).
In this study, GQDs were synthesized from the
coal using modified hummers asisted by
hydrothermal method. To the best of our knowledge,
there is no optical study yet about this coal graphene
quantum dots for optical applications. The GQDs
optical properties were studied by using UV-Vis
spectroscopy and photoluminescence.
2 METHODS
2.1 Materials
Sawahlunto Sijunjung coal graphite powder
(BB900) is obtained from CFM-RC Laboratory.
CPRO WELD commercial graphite powder (KG),
Merck sulfuric acid 98% (H
2
SO
4
), pottasium
permangate (KMnO
4
) and EMSURE sodium
hydroxide (EMSURE) were purchased from Sigma
Aldrich.
584
Dellansyah, ., Gea, S., Andriayani, ., Harahap, M. and Nainggolan, G.
Optical Study of Graphene Quantum Dots from Sawahlunto Coal Graphite.
DOI: 10.5220/0010614000002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 584-586
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
2.2 Synthesis of Graphene Quantum
Dots (GQDS)
BB900 (200 mg) was dispersed in a 100 ml H
2
SO
4
for a few minutes before irradiated by ultrasonic for
2-5 hours. KMnO
4
(1 g) was slowly added to the
mixture under ice batch to keep temperature under
25
o
C and keep until being brownish suspension.
The suspension was heated in hydrothermal
autoclave vessel at 180
o
C for 18-24 hour and cooled
under room temperature. The resultant hydrothermal
was injected slowly to deionized water and added
NaOH until pH become 7 under ice batch condition.
The injected suspension was then filtered using 200
nm porous membrane. The same procedure was also
used to produced GQDs from commercial graphite
powder (GK).
2.3 Characterization
2.3.1 UV-Visible
UV-Vis spectrophotometer (UV 2400 PC Series,
Shimadzu) was used to determined conjugate bond
of GQDs. UV-Vis was carried out in range 200-800
nm.
2.3.2 Photoluminescence
Photoluminescence (AURORA 4000) were used to
determine emission of this GQDs product.
Photoluminescence was carried out in range 200-800
nm with UV-light excitation in 375 nm.
3 RESULTS AND DISCUSSION
Graphene quantum dots (GQDs) in this work were
obtained from Sawahlunto coal graphite (BB900) by
using modified hummers method and assisted by
hydrothermal. Before oxidized with strong oxidator
KMnO
4
,
BB900 was dispersed in strong acid H
2
SO
4
and irradiated using ultrasound to weaken the Van
Der Walls bond (Shin et al., 2014). The oxidized
product was hydrothelmalized to cut graphene oxide
lattice into tiny dots at high temperature (Bak, Kim
and Lee, 2016). Finally, the tiny dots was
characterized by UV-Vis spectroscopy and
photoluminescene spectroscopy.
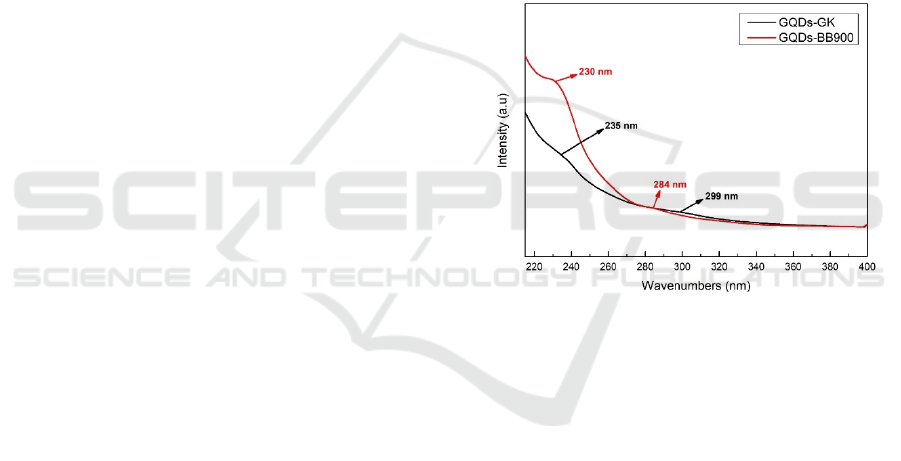
3.1 UV-Vis Spectrocopy Analysis
The conjugate aromatic structure was investigated
by using UV-Vis spectroscopy. Figure 1 showed
that the tiny dots of Sawahlunto coal graphite
(GQDs-BB900) and comercial graphite (GK). Both
tiny dots peaks show that there were no signifikan
friction at the wavelength. GQDs-BB900 and
GQDs-GK had first UV absorptions peaks at 230 nm
and 235 nm respectively. This first absorbance
indicated π–π* C-H sp
2
aromatic transitions (Shin et
al., 2014). The second absorbance was also detected
at 284 nm and 299 nm for GQDs-BB900 and GQDs-
GK. This absorbance confirmed C=O n- π* domains
(Song et al. 2014). The transitions of π–π* aromatic
were generally appeared from 200 to 270 nm and
C=O n- π* transitions were above 260 nm (Chen et
al. 2018). Although there was no significant frictions
in wavelength, the spectra showed significant
spacing in intensity of first absorbances. This
friction confirm that the tiny dots from BB900 had
more conjugated aromatic structure than GK.
Figure 1 UV-vis spectra of graphene quantum dots from
Sawahlunto coal graphite (GQDs-BB900) and commercial
graphite (GQDs-GK)
3.2 Photoluminesnence Analysis
Optical properties of this study were determined by
using photoluminescence (PL) spectrophotometer.
The PL was used to detect the emission of tiny dots
of GQDs as seen in Figure 2. From the Figure, UV
light with excitation at 375 nm was irrariated againts
GQDs-BB900, a strong peak appeared between 412
and 658 nm with maximum intensity at 493 nm.
This maximum intensity indicated that the tiny dots
of Sawahlunto coal graphite emitted cyan emission.
The same excitated UV light was also used against
GQDs-GK, a stronger peak appeared between 412
and 670 nm with maximum intensity at 467 nm. The
maximum intensity indicated that the tiny dots of
commercial graphite emitted blue emission. The
wavelength of blue emission and cyan emission
were 450-485 nm and 485-500 nm respectively
(Bruno and Svoronos, 2005).
Optical Study of Graphene Quantum Dots from Sawahlunto Coal Graphite
585
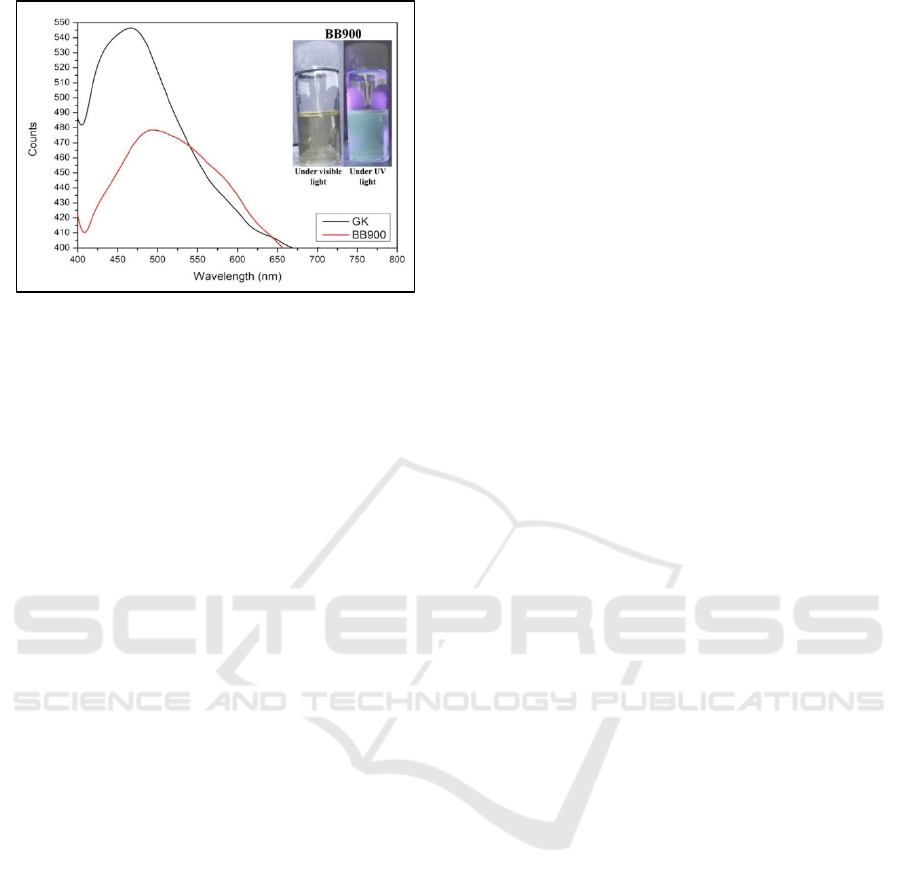
Figure 2 Emission intensity of graphene quantum dots
from sawahlunto coal graphite (GQDs-BB900) and
commercial graphite (GK)
4 CONCLUSION
Graphene quantum dots (GQDs) which was
synthesized from Sawahlunto coal graphite (BB900)
by using Hummers method with hydrothermal
asissted had been done. It has conjugated aromatic
structure and cyan emission which have potential for
optical applications.
ACKNOWLEDGEMENT
The authors thank to KEMENRISTEK DIKTI and
Rector of Universitas Sumatera Utara 2020 for
financial support through DRPM-PTM 2020 with
contract number : 11/AMD/E1/KP.PTNBH/2020.
REFERENCES
Bak, S., Kim, D., Lee, H. 2016. Graphene quantum dots
and their possible energy applications: A review.
Current Applied Physics, [online] 16(9), pp.1192–
1201. Available at:
<http://dx.doi.org/10.1016/j.cap.2016.03.026>.
Bruno, T.J., Svoronos, P.D.N. 2005. CRC handbook of
fundamental spectroscopic correlation charts. CRC
Handbook of Fundamental Spectroscopic Correlation
Charts. CRC Press.
Chen, W., Lv, G., Hu, W., Li, D., Chen, S. and Dai, Z.
2018. Synthesis and applications of graphene quantum
dots: A review. Nanotechnology Reviews, 7(2),
pp.157–185.
Dimiev, A.M.. J.M. 2014. Mechanism of graphene oxide
formation. ACS Nano, 8(3), pp.3060–3068.
Li, X., Rui, M., Song, J., Shen, Z., Zeng, H. 2015. Carbon
and graphene quantum dots for optoelectronic and
energy devices: A Review. Advanced Functional
Materials, 25(31), pp.4929–4947.
Powell, C., Beall, G.W. 2015. Graphene oxide and
graphene from low grade coal: Synthesis,
characterization and applications. Current Opinion in
Colloid and Interface Science, [online] 20(5–6),
pp.362–366. Available at:
<http://dx.doi.org/10.1016/j.cocis.2015.11.003>
Purwandari, V., Gea, S., Wirjosentono, B., Haryono, A.,
Rahayu, S., Hutapea, Y.A. 2020. The exfoliation
process of sawahlunto coal into graphene through the
modified hummer method. Rasayan Journal of
Chemistry, 13(1), pp.593–600.
Shin, Y., Lee, J., Yang, J., Park, J., Lee, K., Kim, S., Park,
Y.,Lee, H. 2014. Mass production of graphene
quantum dots by one-pot synthesis directly from
graphite in high yield. Small, 10(5), pp.866–870.
Song, S.H., Jang, M.H., Chung, J., Jin, S.H., Kim, B.H.,
Hur, S.H., Yoo, S., Cho, Y.H., Jeon, S. 2014. Highly
efficient light-emitting diode of graphene quantum
dots fabricated from graphite intercalation compounds.
Advanced Optical Materials, 2(11), pp.1016–1023.
Sun, Y., Wang, S., Li, C., Luo, P., Tao, L., Wei, Y., Shi,
G. 2013. Large scale preparation of graphene quantum
dots from graphite with tunable fluorescence
properties. Physical Chemistry Chemical Physics,
15(24), pp.9907–9913.
Yan, X.B., Liu, W.W. 2014. Micro-supercapacitors based
on graphene quantum dots. Electrochemical
Conference on Energy & the Environment (ECEE).
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
586
