Lignin Isolation from Oil Palm Empty Fruit
Bunches (OPEFB) by Acidic Method
Saharman Gea
1*
, Amir Hamzah Siregar
2
, Emma Zaidar
2
, Mahyuni Harahap
2
, Yurika Almanda
Perangin-Angin
2
1
Cellulosic and Functional Materials Research Centre Universitas Sumatera Utara, Jalan Bioteknologi Medan, Indonesia
20155
2
Chemistry Department Faculty of Mathematics and Natural Science, Universitas Sumatera Utara, Jalan Bioteknologi,
Medan, Indonesia 20155
Keywords: Oil palm empty fruit bunches, Lignin, FTIR, UV-visible
Abstract: The isolation of lignin from oil palm empty fruit bunches (OPEFB) has been done. Firstly, OPEFB was
immersed in NaOH 2% and 4%. Then, the filtrate was acidified by using H
2
SO
4
5N to obtain the lignin.
SEM morphology of isolated lignin showed rough surfaces. While, chemical structures of the lignin were
analyzed by using FTIR and UV-visible. Aromatic ring vibrations of the phenylpropene (C9) skeleton
appeared at 1600 cm
-1
, 1515 cm
-1
, and 1425 cm
-1
. In addition, the presence of aromatic rings/non-
conjugated phenolic groups in lignin structure was observed at 280 nm absorption.
1 INTRODUCTION
Biomass is the most abundant natural bioresources in
the world. It is known as lignocellulosic material as it
consists of three main molecules namely cellulose,
hemicellulose, and lignin (Ma’Ruf, Pramudono and
Aryanti, 2017). Oil palm empty fruit bunches
(OPEFB) are composed of cellulose (41.3-46.5%),
hemicellulose (25.3-32.5%), and lignin (27.6-32.5%).
OPEFB have been reported for their uses in pulp
production, fertilizers for oil palm plantations, carbon
fibre precursors, and electrospun nanocomposite (Gea
et al., 2020)(Misran et al., 2020). However, the
isolation of lignin from OPEFB is still limited.
Lignin has several functional groups such as
methoxy, carbonyl, carboxyl, and hydroxyl (phenolic
and alcoholic components). The chemical structure of
lignin is presented in Figure 1. The phenolic
compounds in lignin can potentially be used as
macromolecular in toughening agents for epoxy resin,
surfactant, and carbon fibre precursors. Lignin-based
carbon fibre is known to have tensile strength and
modulus of 0.51 GPa and 28.6 GPa respectively
(Baker, Gallego and Baker, 2011).
There are several methods to isolate lignin from
biomass, such as using acid and alkali solvent, ionic
solution
and organic solvent, and alkaline hydrogen
Figure 1. The potential chemical structure of lignin
(Gregory, 2007)
Gea, S., Siregar, A., Zaidar, E., Harahap, M. and Perangin-Angin, Y.
Lignin Isolation from Oil Palm Empty Fruit Bunches (OPEFB) by Acidic Method.
DOI: 10.5220/0010613900002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 581-583
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
581
peroxide (H
2
O
2
) (Ma’Ruf, Pramudono and Aryanti,
2017). The isolation of lignin using acid solvent
method has some advantages, such as low-cost,
efficient production, and low temperature
requirement.
In this study, we extracted lignin from OPEFB
using H
2
SO
4
at room temperature. The isolated
lignin was characterized for its morphology and
chemical structures.
2 EXPERIMENTAL
2.1 Materials
Oil palm empty fruit bunches (OPEFB) were
obtained from PTPN IV Adolina, North Sumatera.
NaOH
(s)
and H
2
SO
4(l)
97% were purchased from
Merck, Germany.
2.2 Isolation and Purification of Lignin
The fibres of OPEFB were immersed in different
concentration of NaOH, such as 2% and 4%. The
immersion process was done at room temperature for
24 h. After that, OPEFB were filtered and acidified
by using 5 N H
2
SO
4
to reach pH 2. The acidified
solution was washed with distilled water and filtered.
The solid parts were collected and centrifuged at
8000 rpm for 5 min. Finally, the isolated lignin was
dried in a vacuum oven at 50
o
C for 4 h. The
products were coded as lignin2% and lignin4%.
2.3 Characterization
2.3.1 FTIR
The functional groups in isolated lignin were
investigated by using FTIR Spectrometer (FTIR,
Nicolet 380, Thermo Scientific, Boston, MA, USA).
The sample was analyzed in a disk form with 100:1
KBr to sample ratio. FTIR instrument was operated
in transmission mode with 400–4000 cm-1
wavelengths, 4 cm-1 resolution, and 100 scans.
2.3.2 UV-Visible
The aromatic rings in lignin were investigated by
using ultraviolet/visible spectrophotometer (UV
1800 series, Shimadzu Scientific Instrument, Kyoto,
Japan). The instrument was operated with
absorbance between 250 and 400 nm wavelengths.
2.3.3 Scanning Electron Microscopy
Sample morphology was analyzed by using Scanning
Electron Microscopy (SEM, Hitachi TM3030, JEOL,
Ltd., Tokyo, Japan) operating at 20 kV. The sample
was first coated with a thin layer of gold before
analysis to reduce charges.
3 RESULTS AND DISCUSSION
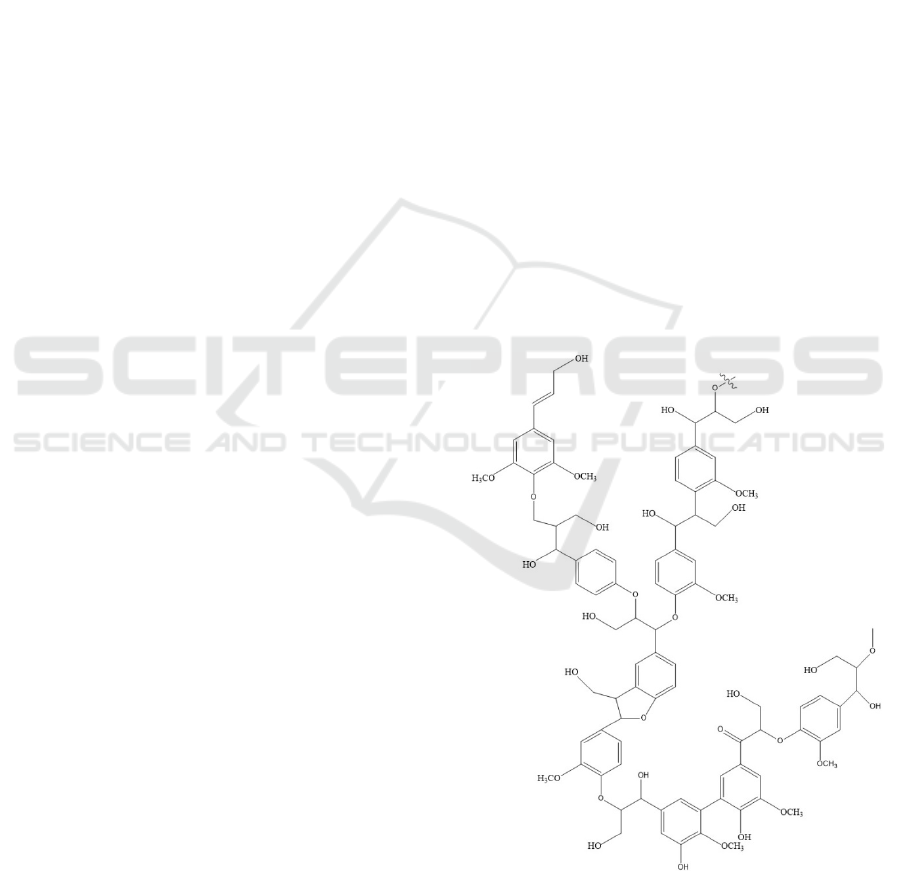
3.1 FTIR Analysis
The functional groups present in the isolated lignin
were analyzed by using FTIR spectral analysis. FTIR
spectra for the polymers are presented in Figure 2.
Aromatic ring vibrations of the phenylpropene (C9)
skeleton could be seen to have appeared at 1600 cm
-
1
, 1515 cm
-1
, and 1425 cm
-1
. The absorption between
3600 and 300 cm
-1
was attributed to hydroxyl groups
in the aromatic and aliphatic structures. In addition,
C-H stretching in methyl and methylene groups, as
well as C-H stretching in aromatic methoxy group
were observed at 2938 cm
-1
and 2885 cm
-1
respectively (Chen et al., 2016; Abdelaziz and
Hulteberg, 2017).
Figure 2 FTIR spectra of isolated lignin immersed in
NaOH 2% and NaOH 4%.
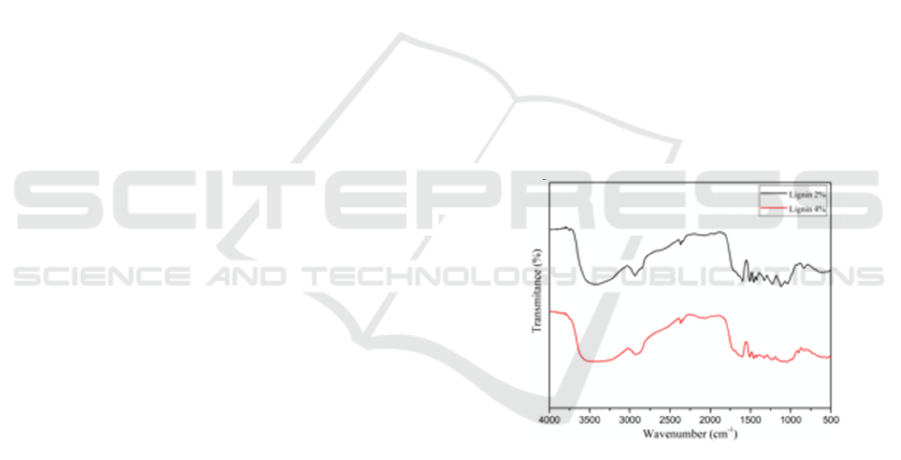
3.2 UV-visible Analysis
UV-visible analysis (Figure 3) was used to
investigate the presence of aromatic rings/non-
conjugated phenolic groups in the structures of
lignin. From Figure 3, there was an absorption at
approximately 280 nm, which indicated aromatic
rings in lignin (Gea et al., 2020).
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
582
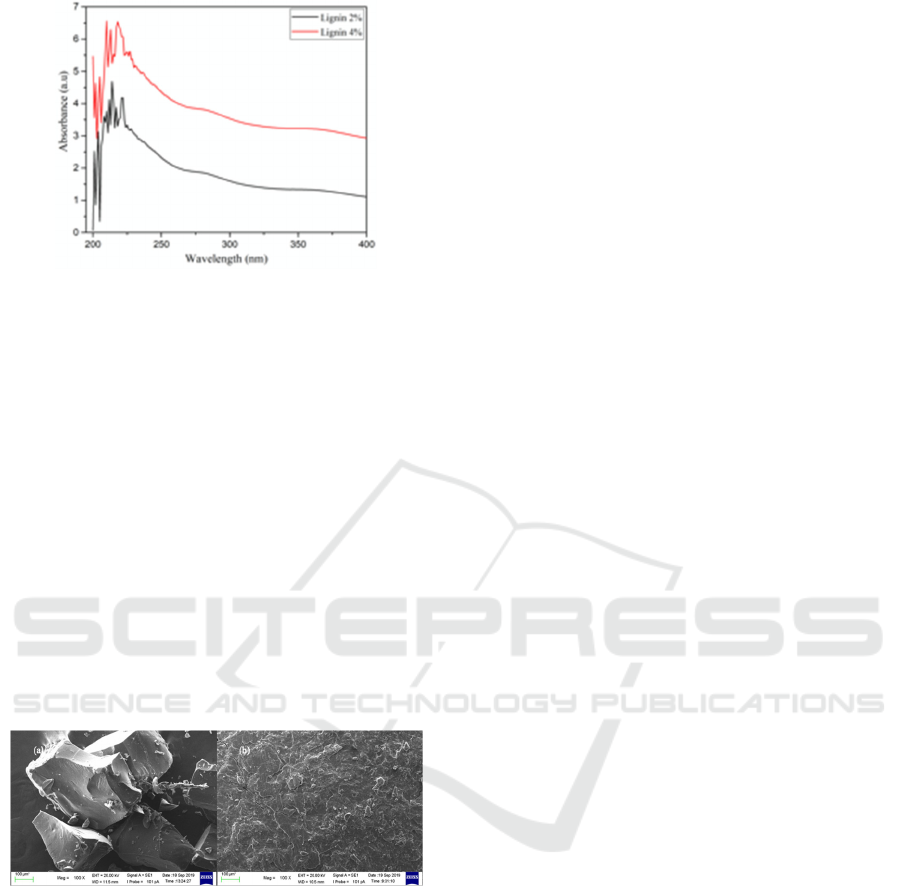
Figure 3. UV-visible of lignin immersed in NaOH 2% and
NaOH 4%.
3.3 Scanning Slectron Microscopy
Morphology
Surface morphology of isolated lignin is shown in
Figure 4. Before analysis, isolated lignin was dried at
80
o
C for 5 h in a vacuum oven to remove moisture
and water content. Then, the sample was coated with
a thin gold to reduce charging during analysis. As
seen in Figure 4, the morphology of isolated lignin
was rough and flaky. This finding result was
different by a previous study that reported lignin
with smooth and uniform morphology in powder
form (van de Pas et al., 2011). These different results
could be caused by different instrument and isolation
procedure used. Furthermore, NaOH 4% produced a
smoother surface morphology than NaOH 2%.
Figure 4. Surface morphology of isolated lignin immersed
in (a) NaOH 2% and (b) NaOH 4%, with magnification of
100x
4 CONCLUSIONS
The isolation of lignin from OPEFB was done by
using acidic solvent (H
2
SO
4
). OPEFB were
immersed in NaOH 2% and 4% before lignin
extraction. FTIR and UV-visible analysis showed
that lignin had aromatic structures. Meanwhile, SEM
analysis confirmed that lignin had rough surface
morphology.
ACKNOWLEDGEMENT
This research was funded by the Indonesian Ministry
of Research and Technology for the supports funds
from DRPM 2019 PDUPT scheme with contract
number 159/UNS.2.3.1/PPM/KP-DRPM/2019.
REFERENCES
Abdelaziz, O.Y., Hulteberg, C. P. 2017. Physicochemical
characterisation of technical lignins for their potential
valorisation, Waste and Biomass Valorization.
Springer Netherlands, 8(3), pp. 859–869. doi:
10.1007/s12649-016-9643-9.
Baker, D. A., Gallego, N. C. and Baker, F. S. 2011 On the
characterization and spinning of an organic- purified
lignin toward the manufacture of low-cost carbon
fiber. doi: 10.1002/app.
Chen, J., Liu C., Wu S., Liang J., Lei M. 2016. Enhancing
the quality of bio-oil from catalytic pyrolysis of kraft
black liquor lignin’, RSC Advances. Royal Society of
Chemistry, 6(109), pp. 107970–107976. doi:
10.1039/c6ra18923g.
Gea, S. Siregar A.H., Zaidar E., Harahap M. 2020.
Isolation and characterisation of cellulose nanofibre
and lignin from oil palm empty fruit bunches’,
Materials, 13(10).
Gregory, A. P. (2007) Green chemistry. Available at:
tp://www.research.uky. edu/images/lignin.jpg.
Ma’Ruf, A., Pramudono, B., Aryanti, N. 2017. Lignin
isolation process from rice husk by alkaline hydrogen
peroxide: lignin and silica extracted’, AIP Conference
Proceedings, 1823(March). doi: 10.1063/1.4978086.
Misran, E., Wiryosentono B., Noor N.M., Gea S.,
Situmorang S.A., Harahap M. 2020. Preparation and
Characterisation of electrospun composite nanofibre
Pplyvinyl alcohol/ nanofibrillated cellulose isolated
from oil palm empty fruit bunches, BioResources,
15(4), pp. 7906–7917. doi: 10.15376/biores.15.4.7906-
7917.
van de Pas, D., Hickson A., Donadson L., Jones G.L. 2011.
Characterization of fractionated lignins polymerized by
fungal laccases, BioResources, 6(2), pp. 1105–1121.
doi: 10.15376/biores.6.2.1105-1121.
Lignin Isolation from Oil Palm Empty Fruit Bunches (OPEFB) by Acidic Method
583
