
In vitro – In vivo Correlation Study of Colon-targeted Metronidazole
Microparticle in Corncob Hemicellulose Capsule
Gabena Indrayani Dalimunthe
1
and Ida Fauziah
2
1
Department of Pharmaceutical Technology, Faculty of Pharmacy,
Universitas Muslim Nusantara Al Washliyah, Medan, Indonesia
2
Department of Biology, Faculty of Science and Technology, Universitas Medan Area, Medan, Indonesia
Keywords: Colon-targeted, Metronidazole, In vitro, In vivo
Abstract: Colon-targeted therapy requires a strategy to keep the medicine pass the stomach and release in the colon,
which is hard to reach by conventional dosage form of metronidazole in gelatin capsule. In this study,
microparticles dosage form of metronidazole which was placed inside corncob-hemicellulose capsule shell
need to be compared with conventionally gelatin capsule of metronidazole preparation to examine whether
it meets medications standards. The aim of this study was to find out the in vitro and in vivo assay
correlation of metronidazole microparticle which covered by corncob hemicellulose capsules. In vitro test
was carried out to observe the profile of differences in the percent release of metronidazole from various
formulations with various media and times. It was performed using a dissolution tester, in an artificial
stomach medium of pH 1.2 for 6 hours in artificial intestinal medium of pH 7.4 for 10 hours, and in artificial
colonic medium of pH 8 for 10 hours. The in vivo test design was conducted using six rabbits. The drug
released in plasma was measured by HPLC using 1% glacial acetic acid solvent in aqua bidest and
methanol-water with a ratio of 80: 20. The test was performed using the cross over design method.
Metronidazole microparticle capsules were administered orally according to the test design (metronidazole
in microparticles and metronidazole in conventional forms). Based on the plotted graph (data retrieval was
started from the drug released in the colon because the drug began to be absorbed at that time), a correlation
value was obtained (R
2
= 0.8785). It can be stated that there is a correlation between the formulations tested
in vitro and in vivo because the correlation value was greater than 0.8, it is assumed to have a correlation.
1 INTRODUCTION
Gelatin capsule has been used for decades in various
therapy as well as for gastrointestinal diseases. The
emerging problems are including the enzymatic
reactions in the upper gastrointestinal tract which
induce premature release of medicine before it
reaches the colon (Nicholas et al. 2011, Lee et al.
2020). Colon Drug Delivery Systems (CDDS) has
become a focus in in drug research and development
resulting in modifications of capsules by using
animal and plant origins material in order to improve
drug bioavailability in the colon (Amidon et al.
2015, Oladzadabbasabadi et al. 2017, Yang et al.
2020). Plant products has become more preferable in
recent years in contrast with products that are made
from animal origin material which could be due to
numerous reasons including consumers views and
beliefs (Cliceri et al. 2018).
The phenomenon has no exception for
medications. Therefore, rapid drug development is
required to enhance efficacy and fit consumers and
patient’s preference. In vitro and in vivo
investigations are paramount in drug development to
confirm the effectiveness which were carried out by
examining the relationship between dissolution and
bioavailability, resulting in the concept of in vitro in
vivo correlation. In the last few years, the concept
and application of in-vitro-in vivo correlation for
pharmaceutical dosage forms has become a major
focus of attention of the pharmaceutical industry,
academics and the regulatory sector (Maity et al.
2016).
From a biopharmaceutical perspective, in vitro-in
vivo (IV-IVC) correlation is a mathematical
prediction that describes the relationship between
the in vitro nature of the dosage form (drug release
rate) and the relevant in vivo response (plasma drug
Dalimunthe, G. and Fauziah, I.
In vitro - In vivo Correlation Study of Colon-targeted Metronidazole Micropar ticle in Corncob Hemicellulose Capsule.
DOI: 10.5220/0010613400002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 561-565
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
561

concentration, urine, amount of drug absorbed).
IVIVC is a tool for the development of drug dosage
forms, since IVIVC can assist in the selection of
drug formulations with suitable and acceptable
dissolution criteria, these predictions can be used as
estimates or substitutes for further bioequivalence
studies (Emami, 2006; Qiu and Duan, 2017)
The in vitro test was carried out to find out the
profile of differences in the percent release of
metronidazole from various formulas with various
mediums and times with a stirring speed of 100 rpm,
medium volume of 900 ml at 370.5°. Meanwhile, in
vivo testing is a test that is carried out using
experimental animals to determine the metabolism
of a compound in the body. Animals used in in vivo
experiments must be from mammals, because the
results can be applied to humans (Chow et al. 2003).
2 MATERIALS AND METHOD
2.1 Materials
Metronidazol (E Merck), NaOH 0.1 N, (E Merck),
CaCl
2
(E Merk), KH
2
PO
4
(E Merk), HCl (E Merck),
NaOCl 5% (E Merk), talcum (Yuanfen), asam
asetate glasial (E Merck), trichoroasetat acid (TCA)
20% , Alkohol 96%, heparin, Metanol for HPLC
(E.Merck), Aquabidestilata (PT. Ikapharmando
Putramas), Metronidazol BPFI (Badan POM RI)
metronidazole tablet (indofarma), all of materials
used in the study are in pro analysis standard (Ahuja,
et al, 2005).
Spectrophotometer (Shimadzu UV-1800),
disintegration tester (Erweka), disolution tester,
HPLC (Agilent 1120 Compact LC), Colom ODS C-
18, solvent container (oberol), vial (agilent), animal
box, vakum pump (Gast DO), sonicator (branson),
paper membrane filter cellulosa nitrate 0,45 μm
(whatman), paper membrane filter nylon 0.45 μm
(whatman), PTFE 02 μm (whatman) ( Muchlisyam,
2014).
2.2 Dissolution Test
Dissolution test was performed using a dissolution
apparatus type 2 (paddle), with 900 mL medium pH
1.2, pH 7.4and pH 8 and temperature of 37 ± 0.5 ° C
with a rotation speed of 100 rpm. At certain time
intervals of 5, 15, and 30 minutes until 600 minuts,
the sample solution was taken 5 mL and measured at
a wavelength of 320 nm (United State
Pharmacopeial convention. 2008; USP, 2009).
2.3 Animal Experiment
Animal test used in this study were male rabbits
weighing 1.5-2 kg, which has been conditioned to
the environment and feeded for 1 week with kale
and carrots during the study. Blood sampling time is
10 minutes after drug administration.
2.4 Plasma Preparation
Rabbits were fasted at least 8 hours prior to the
experiment. Weighed and cleaned fur ears clean.
The blood was taken from 2 male rabbits
approximately 5 ml each, divided into 4 tubes which
had contained 2 drops of heparin, added 2 ml TCA
20%, then centrifuged at 3000 rpm for 10 minutes.
Each supernatant was taken and used as a blanko
and a calibration curve (Kemenkes RI, 2014).
2.5 In vivo Test
The test was conducted using six rabbits. The
administration of metronidazol rabbits with this
method can be seen in Table 1.
Table 1: Microparticle Metronidazole and conventional metronidazole capsules were administered to rabbits using the
cross-over design method (Chilukuri, et al. 2007).
Treatment I
2 Weeks
b
rea
k
Treatment II
Rabbit Dosage
Form
Rabbit Dosage
Form
1 A 1 B
2 A 2 B
3 A 3 B
4 B 4 A
5 B 5 A
6 B 6 A
A= Metronidazole microcapsule capsules, B = Metronidazole capsules (conventional)
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
562
At first, the rabbits were fasted for about 12
hours, then the metronidazole microparticles and the
conventional metronidazole capsules were
administered orally, the dosage forms were
administered based on the procedure according to
Table 3.3. Furthermore, rabbit blood was taken
through the marginal vein at certain time intervals,
namely: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 and
15, hours using a 1.0 mL syringe. The syringe was
initially rinsed with heparin. The blood was put into
a centrifuge tube containing 2 drops of heparin.
Then 1.0 mL of 20% TCA was added to the tube and
vortexed until homogeneous. The tube was put into a
centrifuge and centrifuge at 3000 rpm for 10 minutes
and the supernatant was taken. Each supernatant was
filtered using a 0.2 µm PTFE filter membrane and
the levels were measured using a HPLC device by
injecting 10 µL of supernatant (Ahuja et al, 2005
and Kemenkes RI. 2014).
Rabbits were fasted for 12 hours before orally
administered with FCL-6, the design could be in the
Table 1. After shaving the hair around rabbits ears,
the blood was taken through the marginal vein at
specified time intervals are: 1, 2, 3, 4, 5, 6, 7, 8, 9,
10, 11, 12, 13, 14 dan 15, hours using 1.0 mL
syringe. Syringes rinsed beforehand with heparin.
Blood inserted into the centrifuge tube which
already contains 2 drops of heparin. Then TCA 20%
as much as 1.0 mL tubes were added and shaked
using vortex apparatus until homogeneous. The tube
was centrifuged at 3000 rpm for 10 minutes and the
supernatants were collected. Each supernatant was
filtered using a 0.2 μm PTFE membrane filter and
the metochlopramide concentrations measured using
HPLC instrument by injecting as much as 10 uL
supernatant (Kemenkes RI, 2014).
2.6
Correlation
of In vitro and In vivo
Correlation of in vitro and in vivo was determined
by using a level A correlation that explains the
relationship between the rate of drug release (%
cumulative drug apart) in vitro and speed of drug
release in vivo (plasma drug concentration).
2.7
Analysis
of Blood Plasma Level
Rabbits that have been granted in accordance with
the oral drug bioequivalence trial design that can be
seen in Table 1. At intervals; 1, 2, 3, 4, 5, 6, 7, 8, 9,
10, 11, 12, 13, 14 dan 15, hours, rabbit blood drawn
with the help of 1.0 mL syringe that has been rinsed
with heparin, was transferred to a centrifuge tube
which already contains heparin, and add 2 drops of
20% TCA 1 mL, centrifuged at 3000 rpm for 10
min, the supernatant was taken, filtered with a 0.2
μm PTFE membrane filter and assayed using HPLC.
3 RESULTS AND DISCUSSION
3.1
Correlation
Test
Chilukuri, et al. (2007) stated the correlation
between in vitro assay with in vivo assay can be
explained by using the correlation IVIVC level A
which is a relation between the cumulative percent
of the drug released of in vitro assay and the percent
amount of absorbed drug in blood plasma of in vivo
assay. The release of metronidazol mikroparticle and
konventional metronidazol for the in vitro test.
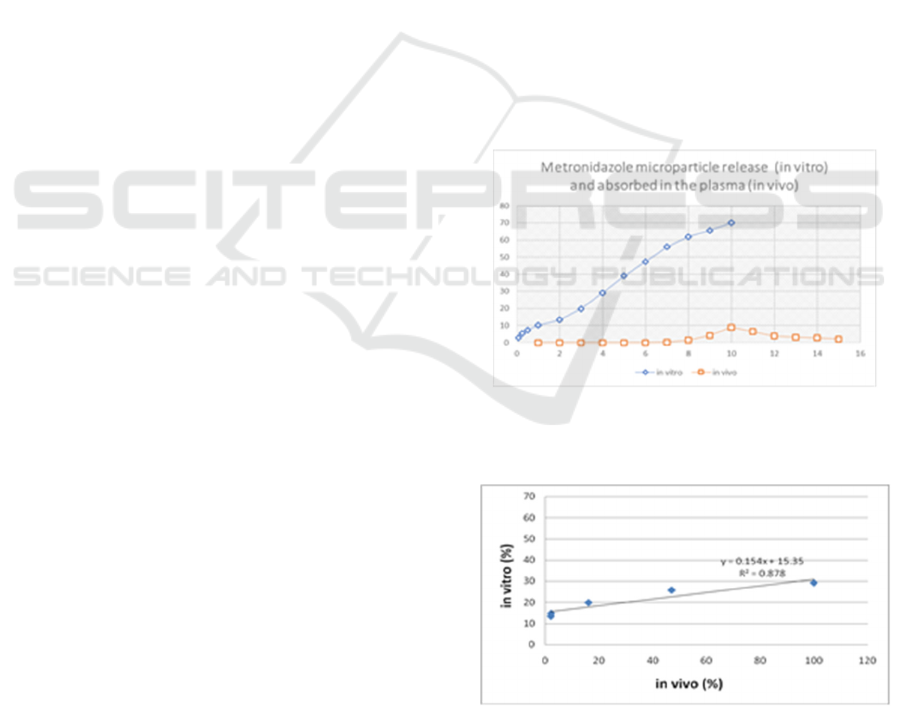
In vitro release of metronidazole and the average
level of metronidazole absorbed in plasma (in vivo)
can be seen in Figure 1. The presence of
metronidazole measured in artificial colon and in the
plasma showed a sustain release pattern. The
optimal in vivo measurement was depicted at 7
hours after administration.
Figure 1: Cumulative percent of metronidazole
microparticles (in vitro) and average percent of
metronidazole microparticles in plasma (in vivo).
Figure 2: In vitro and in vivo correlation of metronidazole
microparticles.
In vitro - In vivo Correlation Study of Colon-targeted Metronidazole Microparticle in Corncob Hemicellulose Capsule
563

Based on the plotted graph (data retrieval was
started from the drug released in the colon because
the drug began to be absorbed at that time), a
correlation value was obtained (R
2
= 0.8785). It can
be stated that there is a correlation between the
formulations tested in vitro and in vivo because the
correlation value was greater than 0.8, it is assumed
to have a correlation (Dalimunthe et al. 2019,
Shargel 1988).
The in vivo test was carried out for 15 hours, but
the new drug was released at 7 hours, which was
about 0.1888 µg / ml, this means that it can be
proven that the capsule is not destroyed in the in
vivo test either in the stomach or in the intestine, but
the intestines have begun to expand, and you can see
that the drug begins to release and there is an
increase in the percentage of drug release at 8 hours.
Based on the reaction kinetics, the in vitro test
(dissolution) shows that the drug follows order 1,
order zero, and higuchi but it tended to be order zero
because the release is relatively constant.
4 CONCLUSION
Corncob hemicellulose capsule considered as
altenate carrier for colon-targeted drug, it was
recorded that the drug release was occurred at pH 8
and 7 hours post oral administration. Therefore, it
has distinctive properties with conventional gelatin
capsule which is vulnerable to stomach acidic
conditions. In vitro in vitro correlation (IVIVC)
study also exhibites a strong relationship which was
indicated by the value of R
2
= 0.8785. It
demonstrated that in vitro dissolution test of
metronidazole microparticle in corncob
hemicellulose capsule is of high relevance for in
vivo assay.
REFERENCES
Ahuja, S., Dong, M., W, Eds. 2005. Handbook of
Pharmaceutical Analysis HPLC edisi 1 United
Kingdom: Elsevier, Inc.,.p. 191-217, 401- 412.
Amidon, S., Brown, J.E., Dave, VS. 2015. Colon-targeted
oral drug delivery systems: Design Trends and
approaches. American Association of Pharmaceutical
Scientist Pharm. Sci.Tech. 16(4) 731-741.
Chilukuri, D.M., Suskara G., Young, D. 2007.
Pharmaceutical Product Development in vitro - in vivo
Corelation, Informa USA, New York. (165):110-
165.
Chow, Shein-Chung, Liu-Pei. 2003. Design and Analysis
of Clinical Trials: Concepts and Methodologies, 3
rd
Edition. Wiley.
Cliceri, D., Spinelli, S., Dinella, C., Prescott, J.,
Monteleone, E. 2018. The influence of psychological
traits, beliefs and taste redponsiveness on implicit
attitudes toward plant-and animal-based dishes among
vegetarians, flexitarians and omnivores. Food Quality
and Preference. Vol 68. 276-291.
Dalimunthe, G.I., Muchlisyam, Urip Harahap, Azizah
Nasution. 2020. The Effect of Variation In The
Amount of Hemicellulose from Corncob (Zea mays L)
and pH On Metronidazole Microparticle Release. IOP
Conf. Series: Journal Of Physics:
Conf.Series.1462.01203.
Dalimunthe, G.I., Muchlisyam Bachri, U. Harahap, A.
Nasution. 2019. Formulation of capsules shell from
corncob hemicellulose combined with isolated sodium
alginate. rasayan Journal of Chemistry: ISSN: 0974-
1496 Rasayan J Chem. Vol. 12(3): 1668-1675.
Emami, J. 2006. In vitro- in vivo correlation: From the
theory to applications. Journal of Pharmacy and
Pharmaceutical Sciences. 9(2): 169-189.
Kemenkes RI. 2014. Farmakope Indonesia. Edisi V.
Jakarta.Departemen Kesehatan RI
Lee, S.H., Bajracharya, R., Min. J.Y., Han, J.W., Park,
B.J., han, H.K. 2020. Strategic approaches for colon
targeted drug delivery: an overview of recent
advancements. Journal of Pharmaceutics. 12(1) 68.
Maity, S., Kundu A., Karmakar S., Biswanath, 2016, In
Vitro and In vivo Correlation of Colon-Targeted
Compression-Coated Tablets, Hindawi Publishing
Corporation. Journal of Pharmaceutics. vol. 2016,
Article.
Muchlisyam, 2014. Corn Cobs Hemicelluloses Isolation
Method Comparison and its Characterization with
Infra Red Spectrophotometry (FTIR) and High
Perfomance Liquid Chrromatography (HPLC).
International Journal of Chem Tech Research CODEN
(USA): IJCRGG ISSN: 0974-4290 vol.6 No.5.pp
3062-3070.
Muchlisyam, Sumaiyah, 2016. Application of corn cobs
hemiselulose microparticle, carrier with insulin as
model. Der Pharma Chemica 8(19): 632: 641.
Muhaimin. 2013, Study of Microparticle Preparation by
The Solvent Evaporation Method Using Focused
Beam Reflectance Measurement (FBRM) Disertation
Nicholas, E.M., Panaganti, S., Prabakaran, l., Jayveera,
KN. 2011. Novel colon spesific drug delivery system:
a review. International Journal of Pharmaceutical
Sciences and Research. Vol 2(10): 2545-2561.
Oladzadabbasabadi, N., Ebadi, S., Nafchi, A.M., Karim,
A.A., Kiahosseini, SR. 2017. Functional properties of
dually/K-carrageenan films: an alternative to gelatin in
pharmaceutical capsules. Carbohydrate Polymers. Vol.
160. 43-51.
Qiu, Y., Duan J.Z. 2017. Chapter 16-In vitro/ in vivo
correlations: Fundamentals, development
considerations and applications. Developing Solid
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
564

Oral Dosage Forms. Pharmaceutical Theory and
Practice. 415-452.
Shargel, L. 1988. Biopharmaceutical and
pharmacokinetics Application., Second Edition.
Interpreter: Sitti Sjamsiah. Surabaya: Airlangga
University Press. p.137, 160-186.
United States Pharmacopoeia. 2007. The National
Formulatory. Edisi Keduapuluh Lima. The United
States Pharmacopoeia Convention XXX. Hal. 277
United State Pharmacopeial convention. 2008. The United
States Pharmacopeia 32
th
. Mack publ.co.easton.
Halaman: 100-103.
USP. 2009. The National Formulary. Edisi ke-32.
Rockville: The United State Pharmacopeia
Convention. .p 3558.
Yang, C., Zhang, Y., Cai, P., Yuan, S., Ma, Q., Song, Y.,
Wei, H., Wu, Z., Wu, Z., Qi, X., 2020. Highly specific
colon targeted transformable capsule containing
indomethacin immediate-release pellets for colon
cancer therapy.
In vitro - In vivo Correlation Study of Colon-targeted Metronidazole Microparticle in Corncob Hemicellulose Capsule
565
