
Isolation and Antioxidant Activity of Phenolic Compound from
Leaves Extract of Clidemia hirta D. Don
Sovia Lenny
1
and Dea Baretta Br Sembiring
1
1
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Medan, North Sumatra 20155, Indonesia
Keywords: Antioxidant, Chromatography, Clidemia hirta, Maceration, Spectroscopy
Abstract: Phenolic compounds have been extracted and isolated to its purity from the leaves extract of Clidemia hirta
D.Don. Extraction procedure included maceration, fractionation, and chromatographic separation of
numerous compounds. Elucidation of purified compound was performed using spectroscopy technique
included UV-Vis, and H-NMR. The results showed that the phenolic compound was categorized as phenolic
acid. Antioxidant activity (IC
50
) of purified phenolic acid based on DPPH assay was 49.9 µg/mL.
1 INTRODUCTION
Wild plants are sources of bioactive compounds
commonly neglected due to the absence of
profounding health and farmacological information.
However, in some region of Indonesia, local people
have already utilized some wild plant species as a
part of ingredient of their traditional remedies
(Tuttolomondo et al, 2014).
Clidemia hirta (L.) D. Don is a wild plant
species native to South America and also distributed
in Australia, South Asia, Sri Lanka, India, East
Africa and other Pacific islands. This herb is widely
used in traditional medicine. In some examples in
Malaysia, the leaves of senduduk bulu are mixed
with saliva and applied as wound-dressing to prevent
bleeding. Moreover, the root decoction of Clidemia
hirta is used by local Malaysian tribes for the
treatment of fever, diarrhea, irritation and bacterial
infections. The boiled water of the leaves and roots
can also treat stomachache and heart disease. In
Brazil, this species is used to treat skin infections
(Lopez et al, 2016).
Two studies highlighted the antibacterial activity
of the leves extract by agar diffusion method,
however no report so far on the bioactive
compounds in the extract. Although the plant has
been described as wild and native plant, they are
also known as invasive shrubs used in folk medicine
to treat several bacterial infections.
Clidermia hirta has antimicrobial activity which
is potential as a source of preservatives in cosmetic
applications. (Abdellaoui et al, 2014). Clidemia hirta
has been extracted in various organic solvents, such
as ethanol, petroleum ether and chloroform which
yielded crude extracts with antiproliferative and
antioxidant activity (Narasimham et al, 2017).
One of the factors causing disease is oxidative
stress which results in cell or tissue damage.
Currently, a viable and safe alternative to synthetic
antioxidants is being developed which are known for
their ability to prevent oxidation (Marianne et al,
2017). Phenolic compounds including phenolic acids
are secondary metabolites found in plants and fungi.
These compounds are produced for protection
against UV rays, insects, viruses and bacterial
infections as well as inhibiting the growth of other
competing plants (allelopathy).
Phenolic acid can be divided into two major
groups, hydroxybenzoic acid and hydroxyinamic
acid (Haleno, 2015; Sousa, 2018). Phenolic acid is a
polyphenol compound that has bioactivity as an
antioxidant, related to the hydroxyl group attached
to the ring structure. These molecules can act in
various roles, i.e. reducing agents, hydrogen donors,
radical scavengers metal chelating superoxides,
superoxide anions and peroxynitrites (Terpinc et al.,
2011).
Other phenolic compounds, namely gallic acid,
apart from having astringent and styptic uses, also
have several bioactivities such as anti-neoplastic,
bacteriostatic, anti-melanogenic and antioxidant
properties (Kim, 2007). This research was conducted
to isolate the phenolic compounds and test the
526
Lenny, S. and Sembiring, D.
Isolation and Antioxidant Activity of Phenolic Compound from Leaves Extract of Clidemia hirta D. Don.
DOI: 10.5220/0010612300002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 526-528
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

antioxidant activity of the leaves extract of Clidemia
hirta (L.) D. Don.
2 MANUSCRIPT PREPARATION
2.1 Materials
Chemicals used for separation and purification
consist of methanol, ethyl acetate, n-hexane, 60 G
(70-230 mesh) silica gel, FeCl
3
, leaves of Clidemia
hirta (L.) D. Don. Tools in this study were
glasswares, column chromatography, TLC GF254
plate 20×20 cm, IR spectroscopy, UV spectroscopy,
1
H-NMR spectroscopy.
2.2 Extraction and Isolation of
Phenolic Compound
Dried powder of Clidemia hirta leaves as much as
1.7 kg was macerated using MeOH to yield crude
MeOH extract (124.64 g). The crude MeOH extract
was further fractionated using hexane and EtOAc to
yield each extract for 37.5 and 10.36 g respectively.
The EtOAc extract was purified under column
chromatography using silica gel as stationary phase
and solvent system using chloroform:MeOH
following gradient of polarity. Each fraction was
checked for its purity on thin layer chromatography
(TLC) by spraying FeCl
3
to indicate any phenolic
compounds. Based on this procedure, we obtained 5
fractions with Fraction-1 (510.5 mg) containing the
highest yield of compound. F1 was further purified
using preparative TLC apparatus. Purified
compound was elucidated using spectroscopy
technique such as UV-Vis, IR, and H –NMR. The
phenolic compound was described following the
description by previous reports.
2.3 Antioxidant Assay
Antioxidative properties of phenolic compound of
Clidemia hirta was assayed based on DPPH radical
scavenging test. Standard solution (DPPH) was
prepared by dissolving 9 mg of compound into 450
mL to obtain a 50 µM solution. The standard
solution was diluted to obtain various concentration
of 100, 50, 25, 12.5, 6.25, and 3.175. Antioxidant
assay was prepared by reacting 0.2 mL of sample
solution into 3.8 mL of 50µM DPPH solution.
Mixture was homogenized for 30 min in dark room.
Absorbance of the solution was checked using UV-
Vis at λ = 516 nm. Antioxidant activity of sample
and positive control was measured using following
formula:
3 RESULTS AND DISCUSSION
3.1 Spectral Analysis of Compound
Purified EtOAc fraction of the leaves extract of
Clidemia hirta (L.) D. Don was yielded as much as
7.4 mg in the form of yellowish white solids. UV-
Vis spectrum (CH
3
OH) showed a peak at 275 nm,
whereas 270–290 nm was determined as the
detection region for phenolic acid compound
(Vijayalakshmi, 2012). FT-IR spectra showed
absorption for hydroxyl group at 3410 cm
-1
, =C-H
group at 2962 cm
-1
, -C-H group at 2924 and 2854
cm
-1
, carbonyl group at 1712 cm
-1
, and aromatic
C=C group at 1612 cm
-1
.
The
1
H-NMR spectrum gave a proton signal with
chemical shift at 7.0 ppm (2H, s, H-2,6) and 3.8 ppm
(3H, s, OCH
3
). These spectrums displayed two
signals at chemical shift δ 7,0 ppm (2H, s) which is
the signal of two aromatic protons at H-2 and H-6
while the chemical shift of δ 3,8 ppm (3H, s) was the
signal of methoxy group. The chemical shift based
on
1
H-NMR resemble the methyl gallate compound
(Ekaprasada, 2009).
3.2 Antioxidant Activity
Determination of antioxidant activity was performed
by measuring the absorbance of remaining DPPH
radical using UV-VIS instrument at 516 nm. The
antioxidant activity was expressed as the percentage
of inhibition of a sample in reacting with radical
solution. Minami et al (1998) grouped the capacity
of antioxidant activity based on IC
50
value. An
antioxidant compound may be classified as very
active, active, and inactive compound if the IC
50
value falls between <10, <100, and >100
respectively. The antioxidant activity of phenolic
acid compound from C. hirta leaves was shown in
Figure 1.
Isolation and Antioxidant Activity of Phenolic Compound from Leaves Extract of Clidemia hirta D. Don
527
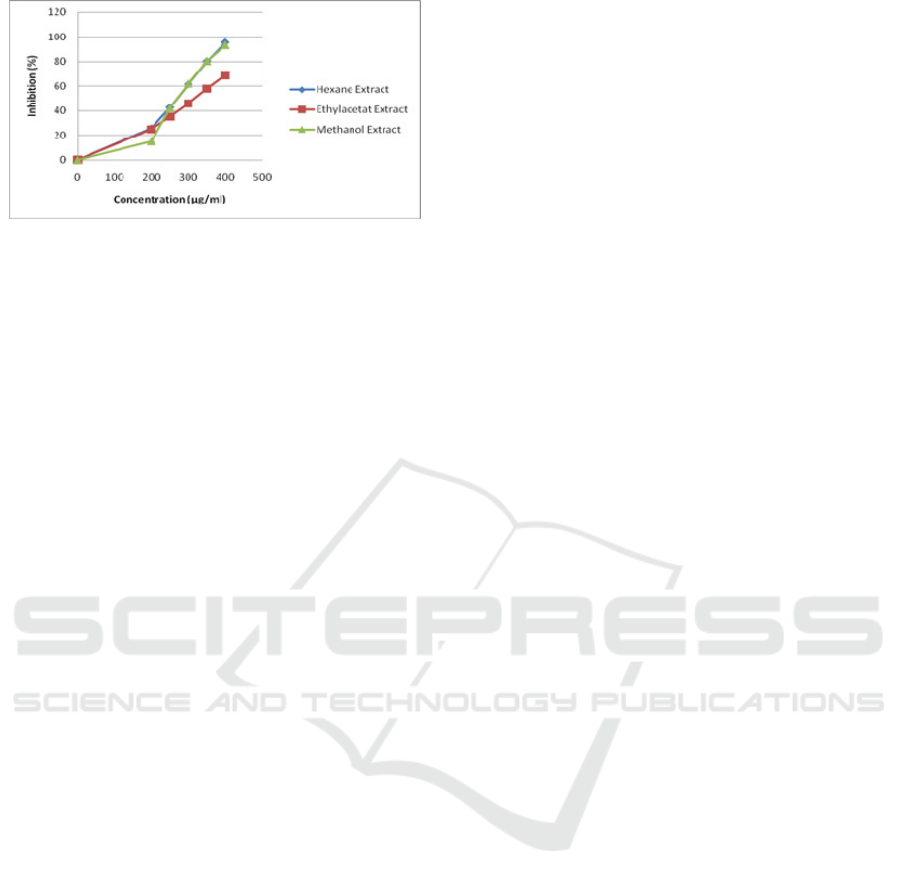
Figure 1. Percentage of DPPH inhibition by various
extracts of Clidemia hirta leaves.
DPPH is a stable free radical as common
standard in determining the antioxidant properties of
a compound or crude extracts. Based on Figure 1,
the IC
50
of MeOH, EtOAc, and hexane extract was
50.8, 49.9, and 88.9 µg/mL. Leaves extract of C.
hirta was classified as an active antioxidant since its
IC
50
< 100. Meanwhile, the EtOAc extract was
regarded as the most prominent antioxidant in this
study.
4 CONCLUSIONS
Purified compound from the leaves extract of
Clidemia hirta was identified as phenolic derivative
or phenolic acid. Antioxidant activity of EtOAc was
determined using DPPH assay showed as IC
50
= 49.9
µg/mL.
REFERENCES
Abdellaoui,S.E., Destandau,E., Krolikiewicz Renimel,l.,
Cancellieri,P., Toribio,A., Jeronimo, Elfakir, C., 2014,
Centrifugal partition chromatography for antibacterial
bio-guided fractionation of Clidemia hirta roots,
Separation and Purification Technology, 123, 221-228
Ekaprasada, M.T., Hazli Nurdin, H., Sanusi Ibrahim, S.,
and Dachriyanus, 2009, Antioxidant Activity of
Methyl Gallate Isolated From The Leaves of Toona
sureni, Indo. J. Chem., 9 (3), 457 - 460
Heleno, S.A., Martins, A., Queiroz, M.J.R.P., Ferreira,
I.C.F.R., 2015, Bioactivity of phenolic acids :
Metabolites versus parent compounds: A review, Food
Chemistry 173:501–513
Kim, Y. J., 2007, Antimelanogenic and antioxidant
properties of gallic acid, Biological and
Pharmaceutical Bulletin, 30, 1052–1055.
Lopez T., Corbin C., Falguieres A., Doussot J.,
Montguillon J., Hagege D., Hano C., Laine E., 2016,
Secondary metabolite accumulation, antibacterial and
antioxidant properties of in vitro propagated Clidemia
hirta L. extracts are influenced by the basal culture
medium. Universite d’Orleans , France
Marianne. H, Salam. Z, Hawraa. S, Kamar. H, Nadine. K,
Hussein. K, 2017, Antioxidant activity of water-
soluble polysaccharide extracted from Eucalyptus
cultivated in Lebanon, Asian Pacific Journal of
Tropical Biomedicine, 7(2), 157-160
Minami, H., Hamaguchi, K., Kubo, M., 1998. A
benzophenone and a xanthone from garcinia
subelliptica, Phytochemistry 49 (6) : 1783-1785
Narasimham D., Bindu Y.H., Cheriyamundath S.,
Raghavan Rahul, Kumari M.K., Chandrasekhar T.,
Madassery J., 2017, Evaluation Of In Vitro
Anticancer and Antioxidant Activities From Leaf
Extracts Of Medicinal Plant Clidemia Hirta, Int J
Pharm Pharm Sci, Vol 9, Issue 4, 149-153
Sousa, M.V., Farias, S.G.G., Castro, D.P., Silva, R.B.,
Silva, D.Y.B.O., Dias, B.A.S., Silva, A.F., Santos,
G.N.L., Matos, D.C.P. and Oliveira, C.V.A., 2018
Allelopathy of the Leaf Extract of Eucalyptus Genetic
Material on the Physiological Performance of Millet
Seeds. American Journal of Plant Sciences, 9, 34-45.
Terpinc, P., Polak, T., Šegatin, N., Hanzlowsky, A., Ulrih,
N. P., Abramovicˇ, H., 2011., Antioxidant properties
of 4-vinyl derivatives of hydroxycinnamic acids, Food
Chemistry, 128, 62–68.
Tuttolomondo, T., Licata, M., Leto. C., Bonsangue,G.,
Gargano, M.L.,Venturella, G., Bella, S.L. 2014
.Popular uses of wild plant species for medicinal
purposes in the Nebrodi Regional Park (North-
Eastern Sicily,Italy).Journal of Ethnopharmacology
157(2014) 21-37
Vijayalakshmi, R., Ravindhran, R., 2012, Comparative
fingerprint and extraction yield of Diospyrus ferrea
(willd.) Bakh. root with phenol compounds (gallic
acid), as determined by uv-vis and ft-ir spectroscopy,
Asian Pacific Journal of Tropical Biomedicine, S1367-
S1371
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
528
