
Antioxidant Activity of Ethanol Extract of Okra (Abelmoschus
esculentus (L.) Moench) and Its Effect on the Expression of p53 in
Breast Cancer Rat Model
Mhd Al Amin Nasution, Syarifah Riska Mela Putri, Salomo Hutahaean, Syafruddin Ilyas,
Widya Sahfitri and Fitri Elizabeth
Department of Biology, Universitas Sumatera Utara, Medan, Indonesia
Keywords: Okra (Abelmoschus esculentus), benzo(α)pyrene -induced Breast Cancer, DPPH Test, p53.
Abstract: Breast cancer is a type of cancer with a high incidence in Indonesia, ranking second highest after cervical
cancer. The search for effective therapeutic agents for breast cancer, including the investigations of natural
agents, still needs to be done. The study was conducted to evaluate the anticancer effect of seed pods
extracts of okra (Abelmoschus esculentus L., Moech) which is traditionally used in the treatment of various
diseases. The objectives of the study were to determine the antioxidant activity of okra seed pods extracts
and evaluate the effect of the extract on tumor severity in the benzo(α)pyrene induced rat breast cancer
model. The experiment was carried out using a completely randomized design, with 5 treatments and 6
replications. These treatments were: 2 control groups (K0 = normal group; K1 = breast cancer model rat due
to benzo(α)pyrene injection), and 3 extract groups (breast cancer model rats were given okra seed pods
extract dose of 150, 300, and 450 mg /kg body weight, respectively). The results showed that okra fruit
ethanol extract has a strong antioxidant activity with IC50 value = 68.79, but the extract has not been able to
trap free radicals due to the induction of benzo(α)pyrene in test animals. Immunohistochemical examination
showed that there was no significant difference and effect between ethanol extract of okra fruit and wild
type p53 expression in test animals induced with benzo(α)pyrene cancer growth compounds.
1 INTRODUCTION
The threat of cancer is increasing along with the
occurrence of lifestyle changes such as smoking,
consumption of fast food, increased pollution, and
ozone layer depletion. Indonesia is a country with a
high cancer burden. The results of early detection
with the Acetic Acid Visual IV (IVA) method to
detect cervical cancer and clinical breast
examination (SADANIS) to detect breast cancer
until 2017 obtained the results of early detection
reaching 1,925,943 or 5.2%. Cancer is a disease
caused by changes in body cells into abnormal cells
and grows out of control.
Cancer treatments such as surgery and
chemotherapy are very expensive, so many
traditional treatments are used as a substitute for
medical treatment, which is herbal treatment by
using plant extracts. One of the plants known to treat
cancer is the okra plant (Abelmoschus esculentus L.
(Moench)). Okra is an important vegetable that is
widespread in Africa, Asia, Southern Europe, and
America. Okra has a role as a source of
carbohydrates, minerals, and vitamins such as
potassium, sodium, magnesium, and calcium
(Khomsug et al. 2010). Okra is a plant that is rich in
flavonoid components as an anti-cancer, maintain
cardiovascular condition, reduces blood sugar levels
and has a series of other properties. Leaves, flowers,
and fruit are parts that contain high flavonoids (Liu
et al. 2017; Van Dam et al. 2013)
The anti-cancer effect of the newly discovered
lectin from the isolation of okra, was investigated in
human breast cancer cells and fibroblast cells where
lectin from okra induced a significant inhibition of
cell growth (63%) in MCF7 cells (Monte et al. 2014).
Research into the anti-cancer mechanism of okra
fruit extracts needs to be done because of the high
incidence and limited information on the anticancer
mechanism of okra fruit ethanol extract. The
anticancer mechanism referred to is one of them
through increased expression of p53 protein, thus, the
Nasution, M., Putri, S., Hutahaean, S., Ilyas, S., Sahfitri, W. and Elizabeth, F.
Antioxidant Activity of Ethanol Extract of Okra (Abelmoschus esculentus (L.) Moench) and Its Effect on the Expression of P53 in Breast Cancer Rat Model.
DOI: 10.5220/0010208300002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 509-513
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
509

presence of p53 will inhibit the function of apoptosis
(Cancer Research Center, 2011). In addition, there is
a relationship between anticancer and antioxidants
because antioxidants can trap free radicals which are
one of the agents that cause breast cancer in humans.
Therefore, this study aims to determine the
antioxidant activity of ethanol extract of okra fruit
(Abelmoshcus esculentus L. (Moench)) and its effect
on the expression of p53 protein in breast cancer
induced by benzo(α)pyrene so it can become a
source of information for those who need to be
developed into an alternative cancer treatment,
especially breast cancer with raw materials derived
from plants.
2 MATERIALS AND METHOD
This research has been conducted at the Pharmacy
Research Laboratory, Organic Chemistry Laboratory,
Laboratory of Structure and Animal Development,
Faculty of Mathematics and Natural Sciences
(FMIPA) and Anatomy Pathology Laboratory,
Faculty of Medicine, University of North Sumatra.
2.1 Research Design
This study used a Completely Randomized Design
(CRD) with 5 treatments and 6 replications so that
the number of samples used was 30 samples, 30
female rats (Rattus sp) Wistar strains.
2.2 Extract Preparation
The okra fruit was obtained from the Growth Center
Laboratory of KOPWIL 1, North Sumatra. After
being collected from the field, the okra fruit that has
been washed clean is dried in an oven at 40
o
C until it
meets the requirements of general moisture content.
Simplisia that is dried and then made into powder
until smooth and sieved with a B
30
sieve. Making
ethanol extract of okra fruit is done by maceration,
ie okra fruit powder is put into a brown bottle and
ethanol is added until submerged and then stirred
and left for 1 night. Take the filtrate and re-soak the
residue with ethanol until a clear filtrate is obtained.
The filtrate obtained was separated with a rotary
evaporator so that a thick extract was obtained.
2.3 Animal Preparation
The experimental animals used were rats (Rattus sp.)
of Wistar strains, female, healthy, aged 8-11 weeks,
with a weight of 200-250 g. Thirty animals obtained
from the North Sumatra Animal Disease
Investigation Center Medan. The animals were
placed in plastic cages with lids made of ram wire,
feed in the form of pellets and drinking water were
given ad libitum. The environment of the cage was
arranged with adequate ventilation and sufficient
light where the light time was 14 hours and the dark
time was 10 hours. Before experimenting, the mice
were adapted in a cage for 7 days. Rat health is
monitored every day.
2.4 Treatment Administration
Breast cancer induction was carried out by injecting
a solution of benzo(α)pyrene to the mammary tissue
of the rat. A single dose of 50 mg/kg body weight
was dissolved in olive oil and injected
subcutaneously. The emergence of tumor mass in
rat breast was observed by palpation for 4 months,
then continued with the administration of the ethanol
extract of okra (Abelmoschus esculentus L., Moech)
seed pods for 15 days. The animals were divided
into 5 groups:
a. A blank control group (K
0
): no treatment.
b. A positive control group (K
1
): single dose of
benzo(α)pyrene
c. Extract I group (P
1
): a single dose of
benzo(α)pyrene + 150 mg/kg body weight
extract.
d. Extract II group (P
2
): a single dose of
benzo(α)pyrene + 300 mg/kg body weight
extract.
e. Extract III group (P
3
): a single dose of
benzo(α)pyrene + 450 mg/kg body weight
extract.
2.5 Immunohistochemistry
The immunohistochemical method used in this study
is an indirect method with a brief procedure:
deparaffination, rehydration, and then immerse the
tissue section in peroxidase blocking solution at room
temperature for 10 minutes. The slides immersed in
a 25°C prediluted blocking serum for 10 minutes,
then incubated in a 25°C anti-p53 wild type primary
antibody for 10 minutes. After washed in phosphate
buffer saline (PBS) for 5 minutes, the slides
incubated in a secondary antibody solution at 25°C
for 10 minutes. The slides washed with PBS for 5
minutes then incubated in a freshly made diamino
benzidine (DAB) solution at 25°C for 10 minutes,
and then counterstained with haematoxylin.
Observations was conducted by calculating the
percentage of cells with p53 expressions and the
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
510
color intensity and the expression area.
Observations were made using a light microscope
with a magnification of 400x (CCRC, 2015).
% Cell expression
100%
Immunoreactive scores = intensity scores x broad scores
(Tan and Putti, 2005)
2.6 Antioxidant Activity Test
The antioxidant activity test of okra extract was
carried out by DPPH free radical trapping method.
A 0.5 mM (200 ppm) solution of DPPH prepared,
and its maximum absorption wavelength was
measured using a UV-Vis spectrophotometer
(400nm-800nm) and obtain a maximum wavelengths
of 516 nm. Ethanol extract of okra fruit prepared in
concentration of 10, 20, 30, 40 and 50 ppm,
incubated for 30 minutes, and measured their
absorbance. The DPPH free radical trapping was
calculated using the formula (Tristantini et al. 2016).
%
damping
. .
.
100%
3 RESULTS AND DISCUSSION
3.1 Antioxidant Activity
The antioxidant activity test of ethanol extract of
okra fruit (Abelmoschus esculentus L. Moench),
showed a decrease in absorbance of DPPH. This is
due to the activity of trapping by the test solution
that is okra fruit ethanol extract. In this process, an
interaction occurs between the extract solution of
okra fruit with DPPH. Ethanol extract of okra fruit
donated 1 hydrogen atom to DPPH so that DPPH
changed into its reduction form (Molyneux, 2004).
Table 1 shows the value of IC
50
determined
through the equation of a linear regression line with
the concentration of the sample as the X-axis and
damping activity as the Y-axis(Utami, 2017).
Table 1: Value IC
50
of the ethanol extract sample of okra
fruit.
Replicati
on
Line equation value
y
IC
50
x
1 y= 0,3682x + 0,8547 50
138.11
2 y= 2,0602x – 20,563 50
34.25
3 y = 1,7585 – 9,8027 50
34
total
206.37
mean
68.79
From the results of data analysis, obtained IC
50
of 68,79 (strong category). This value is inversely
related to the antioxidant activity where the higher
the value of antioxidant activity, the lower the IC
50
value (Molyneux, 2004). The antioxidant activity of
the ethanol extract of okra fruit can also be noted
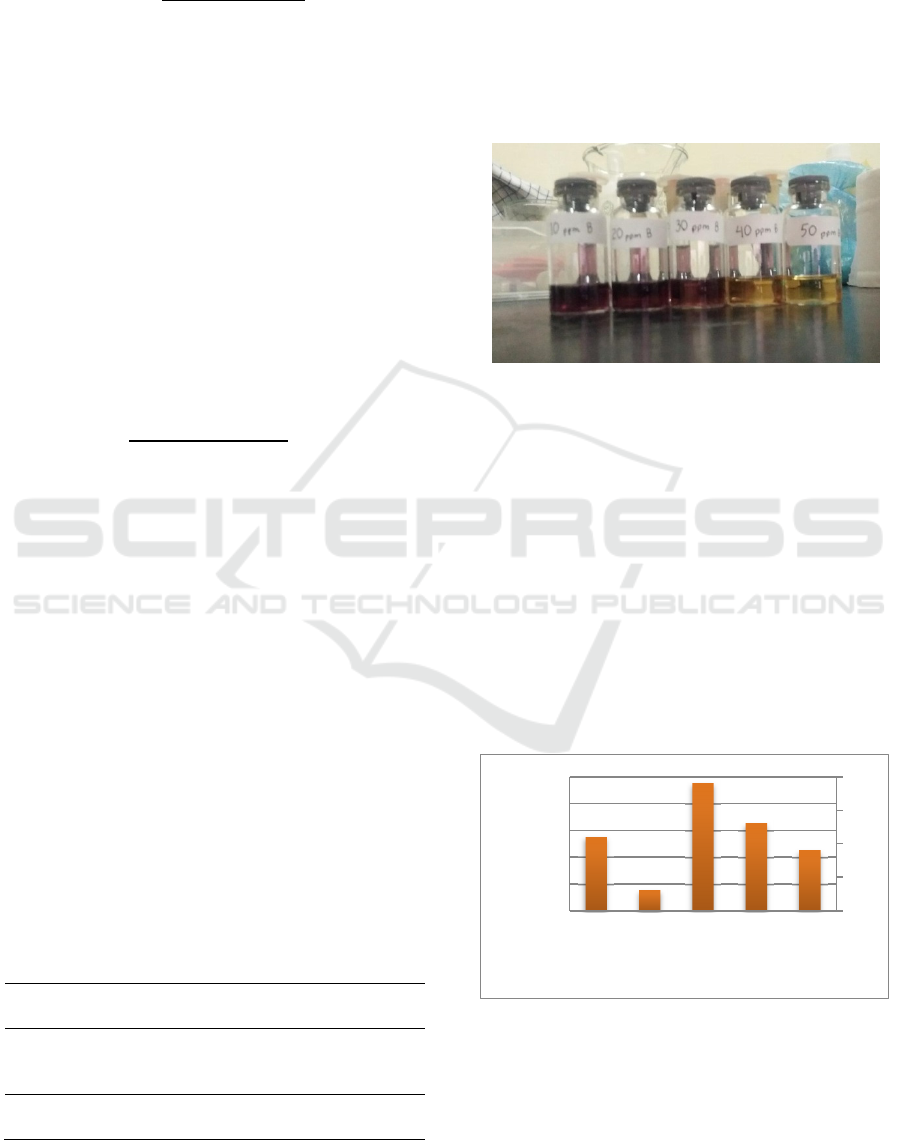
through the change in color of the test sample which
is dark purple when added DPPH will turn yellowish
color if the extract has the damping as seen in the
picture below:
Figure 1: The color change of the test sample.
The presence of antioxidants in plant extracts can
neutralize DPPH radicals by giving electrons to
DPPH, resulting in a change in color from purple to
yellow or the intensity of the purple color of the
solution to be reduced (Molyneux, 2004). This
discoloration causes a decrease in DPPH absorbance
(DPPH purple reduction/ DPPH trapping activity).
3.2 p53 Protein Expression
Quantitative immunohistochemical observations
show that the benzo(α)pyrene control group (K1)
shows almost no wild p53 expression compared to
the normal control group (K0).
Figure 2: Diagram of increasing wild-type p53 expression;
K
0
= normal control, K
1
= benzo(α)pyrene control, P
1
=
benzo(α)pyrene + okra 150 mg/kg BW, P
2
=
benzo(α)pyrene + okra 300 mg/kg BW, P
3
=
benzo(α)pyrene + okra 450 mg/kg BW.
0
20
40
60
80
0
2
4
6
8
10
K0 K1 P1 P2 P3
meanrankekspressionp53
treatment
Antioxidant Activity of Ethanol Extract of Okra (Abelmoschus esculentus (L.) Moench) and Its Effect on the Expression of P53 in Breast
Cancer Rat Model
511

In Figure 2, the lowest percentage of wild p53
expression occurs in the benzo(α)pyrene group (K
1
),
with a mean rank of 1.50 this is due to
benzo(α)pyrene induction can damage the DNA
structure so that many p53 wild type genes mutate
into mutant p53. When DNA damage occurs, p53
holds cells from entering the next phase and gives
DNA time to make repairs, or if the damage is
severe enough, p53 will initiate a cell death program
(apoptosis). The highest p53 expression occurred in
group P1, with a mean rank of 9.50. This increase in
p53 expression will spur apoptosis through 2
mechanisms, namely by increasing Bax protein and
decreasing Bcl-2 protein expression (King, 2000).
In contrast to group P
1
, groups P
2
and P
3
actually
experienced a decrease in wild type expression of
p53 with a mean rank of 6,50 to 4,50. This data
explains that the higher the dose of ethanol extract
given okra can actually reduce the expression of p53
even if the extract dose continues to be increased it
could trigger tumors. It is suspected that there are
other substances in the extract that are interested
when doing maceration with ethanol solvent. Further
research is needed to carry out phytochemical
screening and ascertain the active substance and its
relationship with p53 expression.
In addition to quantitative analysis, qualitative
analysis is also performed to see the expression of
p53 by determining the immunoreactive score of p53
against the p53 antibody staining. Look at the
picture below:
K
0
(normal) K
1
(benzo(α)pyrene)
P
1
(150 mg) P
2
(300 mg)
P
3
(450 mg)
Figure 3: Observation of p53 expression using a light
microscope with a magnification of 400 x.
From the picture above we can see the difference
in color intensity ranging from dark brown, medium
brown, light brown and blue. Likewise with the
colored areas ranging from 0 -> 10%, 10-50% and>
50% by the scoring rules so that the immunoreactive
data obtained in the table below:
Table 2: Immunoreactive score of p53 expression.
Information:
K0 = normal control
K1 = benzo(α)pyrene control
P1 = benzo(α)pyrene + okra 150 mg /kg BW
P2 = benzo(α)pyrene + okra 300 mg / kg BW
P3 = benzo(α)pyrene + okra 450 mg / kg BW
Immunohistochemical observations showed the
presence of wild type p53 protein accumulation.
This accumulation is likely caused by physiological
responses to DNA damage or impaired cell
proliferation in tumor cells (Louis, 1994). The
existence of p53 activation by ethanol extract of
okra fruit (Abelmoshcus esculantus) through p53
stabilization will affect the cell cycle process so that
cells will not experience division and cells will die
due to chromosome condensation that causes
apoptosis, so based on this research it is suspected
that okra fruit extract has activity antimitosis and
proapoptosis in tumor cells. Drugs that have an
antimitotic effect are also thought to have an
antitelomerase effect that can inhibit cell division
and rapid development such as cancer cells and
result in cell death (apoptosis).
Based on the analysis of SPSS data, the
Kolmogorov-Smornov test and the Levene Statistics
test show that the data is abnormally distributed and
not homogeneous because the significance value is
0.00 <0.05 so that the Kruskal-Wallis test is carried
out to determine whether there is an effect of
treatment on the research variables. The results of
data analysis showed there was significant effect of
ethanol extract of Okra (Abelmoschus esculantus L.
Moench) on p53 expression and immunoreactive
scores in breast cancer induced by benzo(α)pyrene.
Group Intensity Large Immunoreactive Information
K
0
+3 2 6 overexpression
K
1
+1 1 1 not expressed
P1 +2 3 6 overexpression
P2 +2 2 4 overexpression
P3 +3 2 6 overexpression
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
512

4 CONCLUSIONS
Based on the results of the study, it can be concluded
that the ethanol extract of okra fruit has a strong
antioxidant activity with an IC
50
value of 68.79, the
extract has been able to trap free radicals due to the
induction of benzo(α)pyrene in test animals. In
addition, there was significant effect of ethanol
extract of okra fruit on wild type p53 expression in
breast cancer rat model.
ACKNOWLEDGEMENTS
We would like to acknowledge the support of
DRPM Ministry of Research and Technology and
the Higher Education Republic of Indonesia which
has provided funding for this research.
REFERENCES
[CCRC] Cancer Chemoprevention Research Centre. 2015.
Prosedur tetap pengecatan imunohistokimia p53.
Downloaded on 10 Agustus 2018, at:
http://www.ccrc.farmasi.ugm.ac.id
[KKRI] Laporan Hasil Riset Kesehatan Dasar (Riskesdas)
Indonesia Tahun 2013. Downloaded on 10 Agustus
2018, at <http://www.depkes.go.id>.
Khomsug, P., Thongjaroenbuangam, W., Pakdeenarong,
N., Suttajit, M., and Chantiratikul P. (2010).
Antioxidative Activities and Phenolic Content of
Extracts from Okra (Abelmoschus esculentus L.).
Research Journal of Biological Sciences. 5(4) : 310-
313.
King, R. J. B. 2000. Cancer Biology, Scound Edition,
Person Education Limited, London.
Liu, H., Sanlong, W., Bin Cai, and Xinsheng, Y., 2004,
Anticancer Activity of Compounds Isolated from
Engelhardtia serrata Stem Bark, Pharmaceutical
Biology, 42: 475–477
Liu, S., J Huang, Meiling Li, Cheng Zhang, Jingjing Zhu,
Yanke Zhao, Xinhong Guo, Jiazhuo Ye. 2017. Study
on Plavonoids and Pectin Contents In Different Okra
(Abelmoschus esculantush) Accession. J Agric Sci
Bot. 1(1)
Louis DN. The p53 gene and protein in human brain
tumors. J neuropathol exp neurol. 1994;53:11-21
Molyneux, P. 2004. The use of the stabel free radical
diphenylpicrylhydrazyl (DPPH) for estimating
antioxidant activity. Songklanakarin Journal Science
Technolog. 26(2) : 211-219.
Monte, lg., santi-gedelhe, T. Reis, LB., Bragonhol, E.,
Priethchs, RF Dellagostin, OA, Elacorda, CA
Conciaco, FR., Pinto. 2014. Lectin of abelmoschus
esculantus (okra) promotes selective antitumor effects
in human breast cancer cell. Biotechnology letter. 36
(3)
Tan, K.B. and Putti, T.C. 2005. Cyclooxygenase-2
Expression in Nasopharyngeal Carcinoma;
Immunohistocemical Findings and Potential
Implication. J Clin Pathol. 58:353-8
Tristanti D; Ismawati A; Pradana B.T; Jonathan JE; 2016.
Pengajuan aktivitas antioksidan menggunakan metode
DPPH pada daun tanjung (mimosa elergi L). Prosiding
Seminar Nasional Teknik Kimia Kejauangan.
Yogyakarta; Universitas Indonesia
Utami, N. H. (2017). Aktivitas Antioksidan dari Ekstrak
Etanol Herba Poguntano (Picria fel-terrae Lour.)
secara In Vitro. Skripsi. Medan: Fakultas Farmasi
Universitas Sumatera Utara. Halaman 26, 34, 43, 48.
Van Dam, RM., Naidoo, N., Landbreg. R. 2013. Dietary
Flavonoids and the Develovment Type 2 Diabetes and
Cardiovascular Diaseas; Revew of Recent Findings.
Curr Opin Lipidol. 24(1)
Antioxidant Activity of Ethanol Extract of Okra (Abelmoschus esculentus (L.) Moench) and Its Effect on the Expression of P53 in Breast
Cancer Rat Model
513
