
The Effect of Ethanol Extract of Okra (Abelmoschus esculentus L.)
Moench) on Tumor Growth in Breast Cancer Rats Model Induced by
Benzo-a-Pyrene
Syarifah Riska Mela Putri
1
, Salomo Hutahaean
1
and Syafruddin Ilyas
1
, Widya Syahfitri
1
and Fitri
Elizabeth
1
1
Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan, Indonesia
Keywords: Okra (Abelmoschus esculentus), BAP-induced Breast Cancer, Angiogenic Effect.
Abstract: Okra plant (Abelmoschus esculentus L. Moench)) is known as a medicinal plant that is traditionally used in
treating various diseases. This study aims to investigate the effect of ethanol extract of okra seed pods on the
growth of tumors in the rat breast cancer model. The experiment was carried out using a completely
randomized design, with 5 treatments and 6 replications. These treatments were: 2 control groups
(K- = normal group; K+ = breast cancer rat model due to benzo-a-pyrene or BAP injection), and 3 extract
groups (breast cancer model rats were given okra extract at dose of 150, 300, or 450 mg/kg BW (body
weight), respectively). The results showed that tumor growth occurred slowly in the first 3 months, but after
that, the growth accelerated marked by an increase in tumor weight and tumor diameter. The average tumor
weight due to BAP induction was 0.6 g, administration of the extracts of doses of 150 and 300 mg/kg BW
gave no significant effect (P>0.05), but unexpectedly in doses of 450 mg/kg BW the average weight was 4.5
g (P<0.05). There is an indication that high-dose extracts stimulate tumor growth. The number of
blood vessels has the same pattern as tumor growth. In the group of rats which were induced by BAP
the number of blood vessels was 6 per visual area, whereas in the extract group the number increased by
9 to 10 vessels. The result suggest that A. esculentus extract may have an angiogenic effect at high doses.
1 INTRODUCTION
Breast cancer is one type of cancer causes a high
mortality rate, especially against women. Breast
cancer ranks second after cervical cancer in
Indonesia. In the United States, breast cancer is the
second leading cause of death in women (after lung
cancer) (Price & Lorraine, 2006). Breast cancer is an
important public health problem, because of its high
mortality and morbidity. Based on research results in
the Jakarta Breast Cancer in 2001 to 2003, of the
2,834 people who had breast lumps examined, 2,229
of them (78%) were benign tumors, 368 people
(13%) were diagnosed with breast cancer and the
rest were infections and breast congenital
abnormalities (Djoerban Z, 2003).
The use of plants for medicinal purposes is
common practice. Okra has been widely used in
various traditional treatments. Even today, many
communities use plants as the main source of
treatment. Okra (Abelmoschus esculentus) is one of
the most widely used plants for treatment. This plant
began to be used to treat various diseases such as
cancer, microbial infections, hypoglycemia,
constipation, urinary retention and inflammation
(Kumar, Patil, Patil, Patil, & Paschapur, 2009;
Tomoda, Shimizu, Gonda, Kanari, & Yamada,
1989).
Okra, Abelmoschus esculentus L. (Moench)
commonly known as "lady’s finger" is cultivated as
an important vegetable crop in tropical, subtropical
and warm temperatures throughout the world.
(Benchasri & Benchasri, 2012; S. Kumar et al.,
2010; Lamont, n.d.; Ndunguru & Rajabu, 2004;
Oyelade et al., 2003). Okra is rich in phenolic
compounds and has high antioxidant activity. Okra
has the potential to prevent several deadly diseases
such as cardiovascular disease, type 2 diabetes,
digestive diseases and some types of cancer
(Gemede et al., 2015). Based on the latest research
on lectins isolated from okra (Abelmoschus
esculentus) tested in human breast cancer and
502
Putri, S., Hutahaean, S., Ilyas, S., Syahfitri, W. and Elizabeth, F.
The Effect of Ethanol Extract of Okra (Abelmoschus esculentus L.) Moench) on Tumor Growth in Breast Cancer Rats Model Induced by Benzo-a-Pyrene.
DOI: 10.5220/0010208200002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 502-508
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

fibroblast cells in the skin, okra has potential as an
anti-tumor (Monte et al., 2014) and dried okra seeds
have the potential to reduce tumor necrosis rates
(Okada et al., 2010). This shows that okra has
potential as an antioxidant contributor and promising
chemopreventive agent for the treatment of diseases
in human
Based on the description above, the research that
will be carried out aims to find the effect of okra as
an anti-tumor by looking at its effect on the growth
of cancer cells by using the right dosage so that it
can be a source of information for those who need to
be developed into an alternative cancer treatment,
especially cancer breast with raw materials derived
from plants.
2 MATERIALS AND METHOD
This research has been carried out at the Laboratory
of Structure and Development of Animals, Faculty
of Mathematics and Natural Sciences, Laboratory of
Organic Chemistry and Natural Materials, Faculty of
Mathematics and Natural Sciences, Pathology
Laboratory of Anatomy, Faculty of Medicine,
University of North Sumatra.
2.1 Experimental Design
This study uses a completely randomized design
(CRD) method which consists of 3 treatments with
different concentrations and 2 treatments as controls.
Both control and treatment each consisted of 6
replications so that there were 30 rats used.
2.2 Making of Okra Fruit Extract
The okra fruit was obtained from the Growth Center
Laboratory of KOPWIL 1, North Sumatra. After
being collected from the field, the okra fruit that has
been washed clean is dried in an oven at 40oC until
it meets the requirements of general moisture
content. Simplisia that is dried and then made into
powder until smooth and sieved with a B30 sieve.
Making ethanol extract of okra fruit is done by
maceration, ie okra fruit powder is put into a brown
bottle and ethanol is added until submerged and then
stirred and left for 1 night. Take the filtrate and re-
soak the residue with ethanol until a clear filtrate is
obtained. The filtrate obtained was separated with a
rotary evaporator so that a thick extract was
obtained.
2.3 Acclimatization of Experimental
Animals
The experimental animals used were rats (Rattus sp.)
Strains of healthy and fertile female Wistar aged 8-
11 weeks with a weight of 200-250 g of 30 animals
obtained from the North Sumatra Animal Disease
Investigation Center Medan. Rats are kept in cages
that are kept clean and feed and drink are done every
day on an ad libitum basis. Handling of experimental
animals by the requirements of the applicable code
of ethics and before the research is conducted, an
application for an Ethical Clearance to the Health
Research Commission of the North Sumatra Region
of Medan is submitted.
2.4 Extract Administration
Carcinogenic induction is carried out by injecting a
solution of benzo (α) pyrene to the subcutaneous
tissue of the Wistar strain rat in the mammary gland.
Benzo (α) pyrene 50 mg/kg BW was dissolved in
olive oil and given a single dose subcutaneously
then observed the emergence of tumor mass in the
breast of the rat by palpation (± 4 months), then
continued with the test substance for 15 days.
The administration of treatment in this research
is
a. negative control: without treatment
b. positive control: administration of Benzo [a]
Pyrene (BAP) at a dose of 50 mg/kg body weight
which will induce the growth of cancer cells in
experimental animals
c. Treatment I: administration of Benzo [a] Pyrene
(BAP) 50 mg/kg BW + ethanol extract of okra
150 mg/kg BW
d. Treatment II: administration of Benzo [a] Pyrene
(BAP) 50 mg/kg BW + ethanol extract of okra
fruit 300 mg/kg BW
e. Treatment III: administration of Benzo [a] Pyrene
(BAP) 50 mg/kg BW + ethanol extract of okra
fruit 450 mg/kg BW
2.5 Making the Histological
Preparations
The histological preparation of the paraffin method
begins with fixation, washing, dehydration, clearing,
infiltration, embedding, slicing, attachment,
deparaffination, staining, closing and labeling
(Suntoro H, 1983).
The Effect of Ethanol Extract of Okra (Abelmoschus esculentus L.) Moench) on Tumor Growth in Breast Cancer Rats Model Induced by
Benzo-a-Pyrene
503
2.6 Hematoxylin Eosin Staining
Hematoxylin-eosin staining is a standard coloring to
determine the general structure of cells and tissues in
an organ. The hematoxylin-eosin staining process
starts from the deparaffination process followed by
the rehydration process using multilevel alcohol then
the preparations werw stained with haematoxylin
and rinsed with distilled water for a few moments.
The preparations are stained again with eosin and
then proceed with the dehydration process. The
preparations were clarified with xylol solution and
continued with the mounting process (Suntoro,
1983).
2.7 Test Parameter Analysis
2.7.1 Visual Morphological Analysis
The morphological observations in this study were
body weight, tumor weight, tumor diameter.
2.7.2 Histological Analysis of Mammary
Gland
The histological part of the mammary gland
observed in Hematoxylin-eosin staining is
vascularization of the mammary gland. Observations
were made by counting the number of blood vessels
formed in the rat breast organs using a microscope
with a magnification of 100x. Observations were
made as many as 5 fields of view.
3 RESULTS AND DISCUSSION
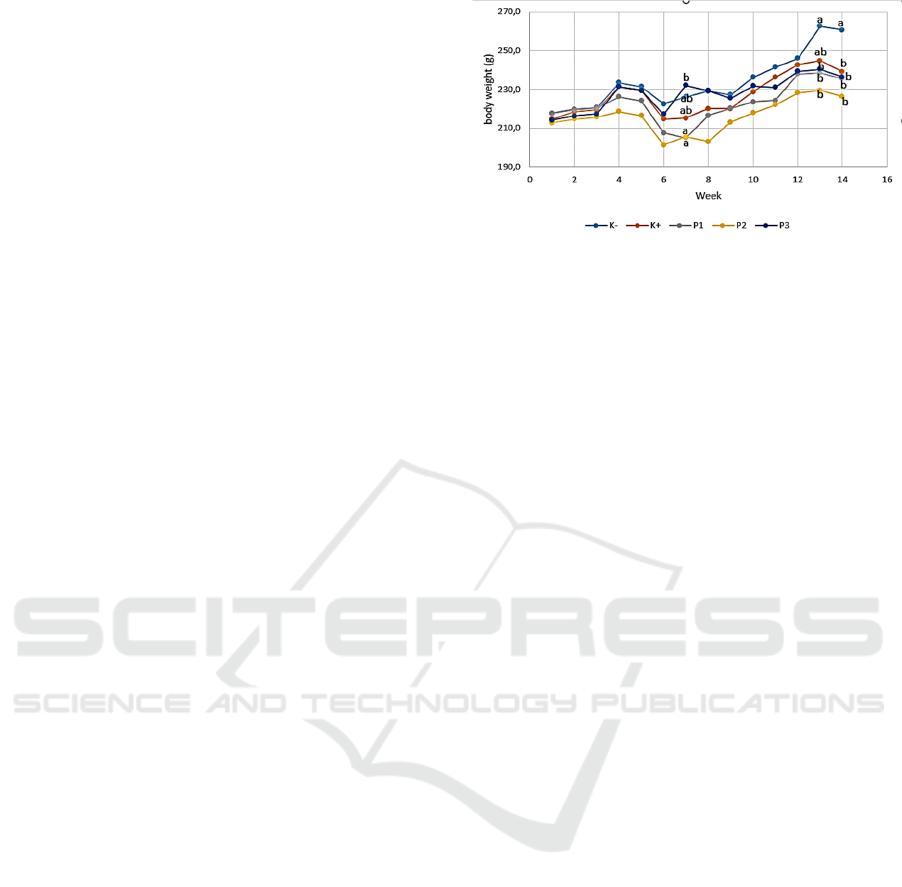
3.1 Body Weight
Based on research that has been done, the
administration of ethanol extract of okra fruit
(Abelmoschus esculentus (L.) Moench) to the
bodyweight of rats, the highest weight was the
negative control group in which the weight was
261.6 g at week 13 and the lowest body weight was
P2 group which was 201.3 g at week 6. The results
of observations of body weight of rats (Rattus sp.)
can be seen in Figure 1.
Figure 1: Effect of Okra Fruit Ethanol Extract on Body
Weight of Rats (Rattus sp.) K- = Negative Control (mice
not trained); K + = Positive Control (BAP distribution);
P1, P2 and P3 = Treatment with ethanol extract
concentrations of 150 mg/kg okra fruit, 300 mg/kg, and
450 mg/kg; unit in grams (g).
Statistical analysis showed that at week 6 the
body weight of the P3 treatment group was
significantly different (P <0.05) to the body weight
of the treatment group P1 and P2 rats but not
significantly different to the positive and negative
control groups. At the 13th week during the
administration of okra fruit ethanol extract, an
increase in body weight of rats in which the negative
control group was significantly different (P <0.05)
with the treatment groups P1, P2, P3 and not
significantly different from the positive control
group. At the 14th week after administration of okra
fruit ethanol extract there was a decrease in body
weight in which the negative control group was
significantly different (P <0.05) with positive control
and treatment groups P1, P2 and P3.
Week 4 to week 10 there is an increase and
decrease in body weight that is volatile.
Inflammation in mice after BAP administration does
not affect the body weight of mice but the
physiological conditions of rats and other external
factors such as weather can affect the body weight of
mice. The size of mammary gland tumors in mice
affects the body weight of mice. It can be seen in
Figure 1 that there was a gain in weight at week 13
due to a significant increase in tumor size from the
previous weeks so that the tumor mass affected the
body weight of mice. In recent years, there have
been reports showing that mice became fat in
carcinogenicity studies and tumor growth in these
mice. Selection, disease control, improved diet, and
better control of environmental conditions led to an
increase in body weight and life span of mice used
in long-term toxicity studies over the past 2 decades
(Rao et al., 1990). There was a decrease in body
weight of rats in the 14th week after giving ethanol
extract of okra fruit due to the size of the tumor that
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
504
began to shrink so that the tumor mass also
decreased which would affect the body weight of the
rat. Anorexia and weight loss are part of end-stage
cancer syndrome which is a major cause of
morbidity and mortality in cancer (Johnen et al.,
2007).
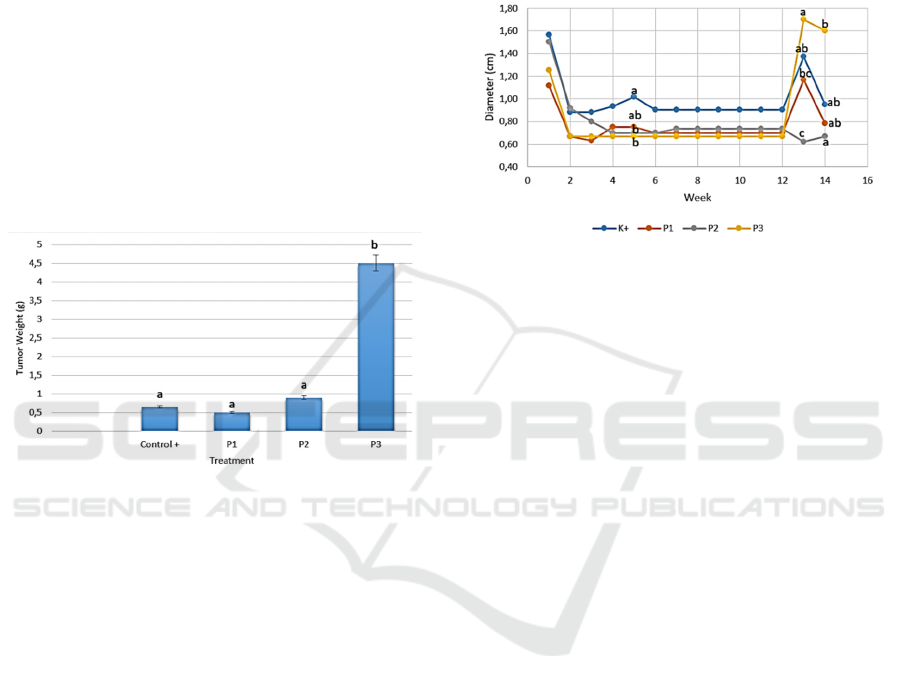
3.2 Tumor Weight of Mammary Gland
Based on research that has been done, the
administration of ethanol extract of okra fruit
(Abelmoschus esculentus (L.) Moench) on mammary
gland tumor weight (Rattus sp.), it can be seen that
the highest tumor weight was the P3 group in which
the weight was 4.51 g and the lowest was the P1
group which was 0.5 g. The results of observations
of mammary gland tumor weight (Rattus sp.) Can be
seen in Figure 2.
Figure 2: Effect of Okra Fruit Ethanol Extract on Tumor
Weight of Mammary Gland of Rats (Rattus sp.) K- =
Negative Control (mice not trained); K + = Positive
Control (BAP distribution); P1, P2 and P3 = Treatment
with ethanol extract concentrations of 150 mg/kg okra
fruit, 300 mg/kg, and 450 mg/kg; unit in grams (g).
The results of statistical analysis showed that the
tumor weight of the P3 group was significantly
different (P <0.05) to the weight of the positive
tumor group, the treatment groups of P1 and P2. The
increase in tumor weight is caused by the continued
division of cells. Proto-oncogenes are genes that
help cells grow normally. When proto-oncogenes
mutate (change) or there are too many copies, cells
grow out of control. This can cause cancer. When
this gene changes, it no longer suppresses the growth
of abnormal cells and cancer is more likely to
develop (American Cancer Society, 2018).
3.3 Tumor Diameter of Mammary
Gland
Based on research that has been done, the
administration of ethanol extract of okra fruit
(Abelmoschus esculentus (L.) Moench) on mammary
gland tumor diameter (Rattus sp.), it can be seen that
the highest tumor diameter was the P3 group at week
13 which was 1.63 cm and the lowest tumor
diameter was the P2 group at 13 weeks which a
length was 0.64 cm. The results of observations on
the diameter of mammary gland tumors can be seen
in Figure 3.
Figure 3: Effect of Okra Fruit Ethanol Extract on Tumor
Diameter of Mammary Gland of Rats (Rattus sp.) K- =
Negative Control (mice not trained); K+ = Positive
Control (BAP distribution); P1, P2 and P3 = Treatment
with ethanol extract concentrations of 150 mg/kg okra
fruit, 300 mg/kg, and 450 mg/kg; unit in centi meter (cm).
Statistical analysis showed that at week 5th the
diameter of the mammary gland tumor mice in the
positive control group was significantly different (P
<0.05) to the diameter of the mammary gland tumor
in the treatment group P2 and P3 but it was not
significantly different in the treatment group P1.
There was a decrease in the diameter of the
mammary gland in the second week after BAP
administration because at this stage the swollen
mammary glands due to inflammation that formed
after injection had begun to shrink. Inflammation
can cause tumor growth and development through its
influence on cell proliferation, tumor survival and
metastasis (Buckland et al., 2014).
In the following weeks, tumors began to form
marked by the presence of thickening in the skin of
the mammary gland of mice. The primary sign of a
tumor in the mammary gland is the irregular border
of the tumor due to the infiltration process into the
surrounding tissue or unclear boundary (comet sign)
and also changes in tumor mass both in size,
consistency, and shape. The only way to diagnose
gold (gold standard) in breast cancer is by
histopathological examination, with this type of
histology known (type), sub-type and cellular
grading and core grading (Ramli, 2015). One of the
histological examination of tumors is the
determination of Ag-Nor grains in which the
The Effect of Ethanol Extract of Okra (Abelmoschus esculentus L.) Moench) on Tumor Growth in Breast Cancer Rats Model Induced by
Benzo-a-Pyrene
505
Nucleolar Organizing Region (NOR) is a place of
ribosomal biogenesis in the cell nucleus whose
numbers increase with the increase in the activity of
cell protein biosynthesis (Hutahaean et al., 2009).
At week 12 there was a very significant change in
tumor size and metastasis that was marked by the
growth of tumors in other organs both in other
mammary glands and in the abdomen of mice. At
the 13th week during the administration of okra fruit
ethanol extract, a decrease in the diameter of the
tumor in mammary glands occurred. Lectins
contained in okra fruit can inhibit the growth of
mammary gland tumor cells in vitro (Monte et al.,
2014).
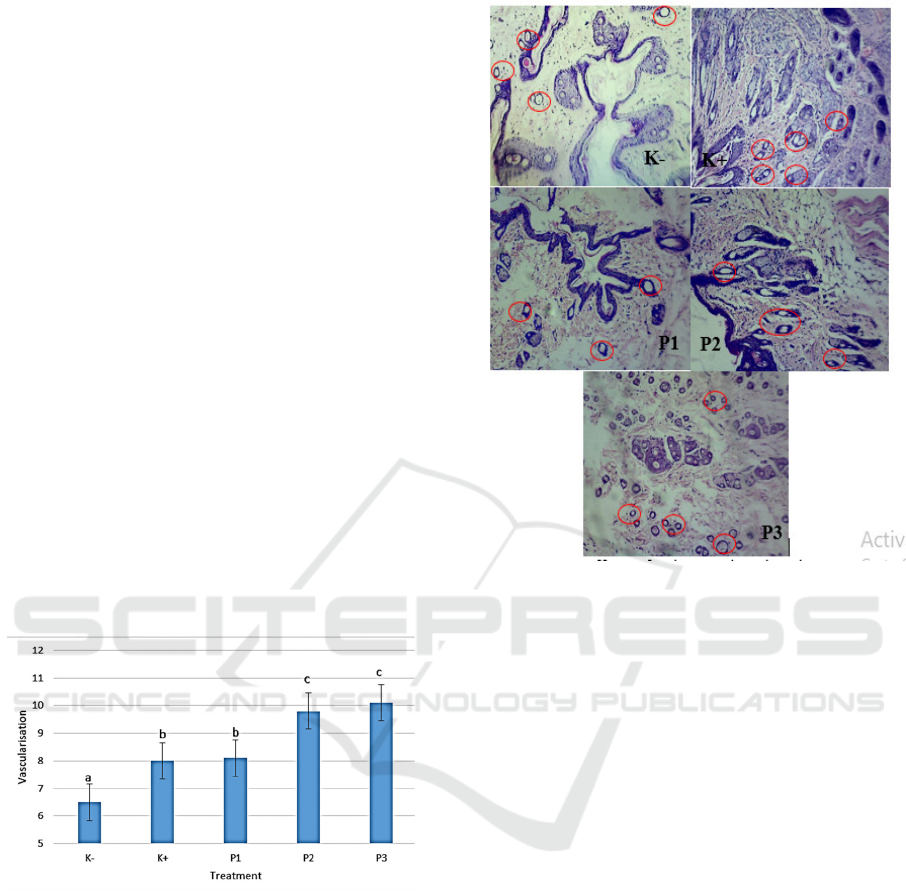
3.4 Tumor Vascularisation of
Mammary Gland
Based on research that has been done, the
administration of ethanol extract of okra fruit
(Abelmoschus esculentus (L.) Moench) against
vascularisation in mammary gland tumor can be
seen that the highest number of blood vessels is
found in the P3 group which amounted to 10.1 and
the lowest blood vessel is the negative control group
which amounted to 6.5. The results can be seen in
Figure 4.
Figure 4: Effect of Okra Fruit Ethanol Extract on Tumor
Vascularisation of Mammary Gland of Rats (Rattus sp.)
K- = Negative Control (mice not trained); K + = Positive
Control (BAP distribution); P1, P2 and P3 = Treatment
with ethanol extract concentrations of 150 mg/kg okra
fruit, 300 mg/kg, and 450 mg/kg.
Figure 5: Effect of Okra Fruit Ethanol Extract on Tumor
Vascularisation of Mammary Gland of Rats (Rattus sp.)
K- = Negative Control (mice not trained); K + = Positive
Control (BAP distribution); P1, P2 and P3 = Treatment
with ethanol extract concentrations of 150 mg/kg okra
fruit, 300 mg/kg, and 450 mg/kg. Vascularisation is
indicated by a red circle.
The analysis showed that the number of blood
vessels formed in the negative control group was
significantly different (P <0.05) for all treatment
groups. This is in line with the tumor mass of
mammary glands, where the greater the tumor mass,
the more blood vessels are formed. One of the
secondary features of the presence of a tumor in the
mammary gland is characterized by an increase in
blood vessels (Ramli, 2015). Vascularization or also
called angiogenesis is the formation of new blood
vessels originating from existing blood vessels.
Under pathological conditions, angiogenesis is
needed in the growth process of solid tumors and the
process of metastasis (Hicklin & Ellis, 2005; Lee S.
Rosen, 2002). Tumors require angiogenesis to grow
above 1-2 mm
3
in size (Lee S. Rosen, 2002).
Angiogenesis is needed for oxygen supply, nutrients,
growth factors and hormones, proteolytic enzymes,
influencing hemostatic factors that control
coagulation and fibrinolytic systems and the spread
of tumor cells to distant sites (Hicklin & Ellis,
2005).
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
506

4 CONCLUSIONS
The results showed that tumor growth occurred
slowly in the first 3 months, but after that, the
growth accelerated marked by an increase in tumor
weight and tumor diameter. The average tumor
weight due to BAP induction was 0.6 g,
administration of the extracts of doses of 150 and
300 mg/kg BW gave no significant effect (P>0.05),
but unexpectedly in doses of 450 mg/kg BW, the
average weight was 4.5 g (P<0.05). There is an
indication that high-dose extracts stimulate
tumor growth. The number of blood vessels has
the same pattern as tumor growth. In the group
of rats which were induced by BAP the number of
blood vessels was 6 per visual area, whereas in the
extract group the number increased by 9 to 10
vessels. The result suggest that A. esculentus extract
may have an angiogenic effect at high doses.
ACKNOWLEDGEMENTS
We would like to acknowledge the support of
DRPM Ministry of Research and Technology and
the Higher Education Republic of Indonesia which
has provided funding for this research.
REFERENCES
American Cancer Society. (2018). About Breast Cancer.
Benchasri, S., & Benchasri, S. (2012). Okra as a Valuable
Vegetable of the World. Ratar. Povrt, 49, 105–112.
https://doi.org/10.5937/ratpov49-1172
Buckland, G., Travier, N., & Agudo, A. (2014). The role
of diet, weight control and physical activity in breast
cancer survivors. Breast Cancer Management, 3(6),
495–503. https://doi.org/10.2217/bmt.14.38
Djoerban Z. (2003). No TitleKanker Payudara:Yang
Penting dan Perlu Diketahui. Medicinal: Jurnal
Kedokteran, 4(2).
Gemede, H. F., Ratta, N., Haki, G. D., Woldegiorgis, A.
Z., & Beyene, F. (2015). Nutritional Quality and
Health Benefits of Okra (Abelmoschus esculentus): A
Review. J Food Process Technol, 6(6), 458.
https://doi.org/10.4172/2157-7110.1000458
Hicklin, D. J., & Ellis, L. M. (2005). Role of the vascular
endothelial growth factor pathway in tumor growth
and angiogenesis. Journal of Clinical Oncology :
Official Journal of the American Society of Clinical
Oncology, 23(5), 1011–1027.
https://doi.org/10.1200/JCO.2005.06.081
Hutahaean, S., Mangkoewidjojo, S., Sagi, M., & Asmara,
W. (2009). Prosiding Seminar Nasional Penelitian,
Pendidikan dan Penerapan MIPA, Fakultas MIPA.
Johnen, H., Lin, S., Kuffner, T., Brown, D. A., Tsai, V.
W.-W., Bauskin, A. R., Wu, L., Pankhurst, G., Jiang,
L., Junankar, S., Hunter, M., Fairlie, W. D., Lee, N. J.,
Enriquez, R. F., Baldock, P. A., Corey, E., Apple, F.
S., Murakami, M. M., Lin, E.-J., … Breit, S. N.
(2007). Tumor-induced anorexia and weight loss are
mediated by the TGF-β superfamily cytokine MIC-1.
Nature Medicine, 13(11), 1333–1340.
https://doi.org/10.1038/nm1677
Kumar, R., Patil, S., Patil, M. B., Patil, S. R., &
Paschapur, M. S. (2009). Isolation and Evaluation of
Disintegrant Properties of Fenugreek Seed Mucilage.
International Journal of PharmTech Research
CODEN, 1(4), 982–996.
Kumar, S., Dagnoko, S., Haougui, A., Ratnadass, A.,
Pasternak, D., & Kouame, C. (2010). Okra
(Abelmoschus spp.) in West and Central Africa:
Potential and progress on its improvement. African
Journal of Agricultural Research, 5(25), 3590–3598.
https://doi.org/10.5897/AJAR10.839
Lamont, W. J. (n.d.). Okra-A Versatile Vegetable Crop.
Lee S. Rosen, M. (2002). Clinical Experience With
Angiogenesis Signaling Inhibitors: Focus on Vascular
Endothelial Growth Factor (VEGF) Blockers. Cancer
Control: Journal of the Moffitt Cancer Center, 9(2).
https://doi.org/10.1177/107327480200902S05
Monte, L. G., Santi-Gadelha, T., Reis, L. B., Braganhol,
E., Prietsch, R. F., Dellagostin, O. A., e Lacerda, R.
R., Gadelha, C. A. A., Conceição, F. R., & Pinto, L. S.
(2014). Lectin of Abelmoschus esculentus (okra)
promotes selective antitumor effects in human breast
cancer cells. Biotechnology Letters, 36(3), 461–469.
https://doi.org/10.1007/s10529-013-1382-4
Ndunguru, J., & Rajabu, A. C. (2004). Effect of okra
mosaic virus disease on the above-ground
morphological yield components of okra in Tanzania.
Scientia Horticulturae, 99, 225–235.
https://doi.org/10.1016/S0304-4238(03)00108-0
Okada, Y., Okada, M., & Sagesaka, Y. (2010). Screening
of Dried Plant Seed Extracts for Adiponectin
Production Activity and Tumor Necrosis Factor-Alpha
Inhibitory Activity on 3T3-L1 Adipocytes. Plant
Foods for Human Nutrition, 65(3), 225–232.
https://doi.org/10.1007/s11130-010-0184-2
Oyelade, O. J., Ade-Omowaye, B. I. O., & Adeomi, V. F.
(2003). Influence of variety on protein, fat contents
and some physical characteristics of okra seeds.
Journal of Food Engineering, 57(2), 111–114.
https://doi.org/10.1016/S0260-8774(02)00279-0
Price, S. A., & Lorraine, M. W. (2006). Patofisiologi :
Konsep Klinis Proses-Proses Penyakit. (6th ed.).
EGC.
Ramli, M. (2015). UPDATE BREAST CANCER
MANAGEMENT DIAGNOSTIC AND
TREATMENT Muchlis Ramli. Majalah Kedokteran
Andalas, 38.
Rao, G. N., Haseman, J. K., Grumbein, S., Crawford, D.
D., & Eustis, S. L. (1990). Growth, Body Weight,
Survival, and Tumor Trends in F344/N Rats During an
Eleven-Year Period" (Vol. 18, Issue 1).
The Effect of Ethanol Extract of Okra (Abelmoschus esculentus L.) Moench) on Tumor Growth in Breast Cancer Rats Model Induced by
Benzo-a-Pyrene
507

Suntoro H. (1983). Metode Pewarnaan. Bhratara Karya
Aksara.
Tomoda+, M., Shimizu, N., Gonda, R., Kanari, M., &
Yamada, H. (1989). Anticomplementary and
hypoglycemic activity of Okra and Hibiscus
mucilages*. In Carbohydrate Research (Vol. 190).
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
508
