
Synthesis of 6-alyl-8-methoxy-3-propyl-1,3-benzoxazine and 4-alyl-6-
(dimethylamino) methyl-2-methoxy phenol from Eugenol through
Mannich Reaction and Antibacterial Activity Test
Sabarmin Perangin-angin and Sajidah Chairi
Department of Chemistry, Universitas Sumatera Utara, Medan, Indonesia
Keywords: Antibacterial, Eugenol, Mannich Reaction, 4-alyl-6-(dimethylamino)methyl-2-methoxy phenol, 6-alyl-8-
methoxy-3-propyl-1,3-benzoxazine
Abstract: 6-alyl-8-methoxy-3-propyl-1,3-benzoxazine and 4-alyl-6- (dimethylamino) methyl-2-methoxy phenol
compounds have been synthesized through the Mannich reaction. The 6-alyl-8-methoxy-3-propyl-1,3-
benzoxazine compound was synthesized by reacting eugenol, 37% formaldehyde, and propylamine under
conditions of reflux with ethanol solvents at 78ºC for 6 hours obtained compounds of 6,116 grams
(82.54%). The formation of 6-alyl-8-methoxy-3-propyl-1,3-benzoxazine compound characterized by FT-IR
obtained CN stretching vibration at wave number 1242.16 cm-1 and peak molecular ion m/e 247 through
GC-MS analysis. The 4-alyl-6- (dimethylamino) methyl-2-methoxy phenol compound was synthesized by
reacting eugenol, 37% formaldehyde, and 40% dimethylamine under reflux conditions with ethanol solvents
at 78ºC for 90 minutes and obtained compounds of 5,728 grams (86.39%). 4-alyl-6- (dimethylamino)
methyl-2-methoxy phenol compound characterized by FT-IR obtained CN and OH stretch vibrations at
wave numbers 1242.16 cm-1 and 3410.15 cm-1 and molecular ion peaks m/e 221 through GC-MS analysis.
Then the two antibacterial activity tests were carried out on the two compounds using Streptococcus mutans
and Escherichia coli bacteria with various concentrations of 10%, 20%, and 30%. The results obtained
showed that the 6-alyl-8-methoxy-3-propyl-1,3-benzoxazine and 4-alyl-6- (dimethylamino) methyl-2-
methoxy phenol compounds were classified as strong antibacterial.
1 INTRODUCTION
Eugenol is a phenolic compound that has several
functional groups such as allyl, hydroxide and
methoxy. With the existence of these functional
groups, the compound eugenol can be transformed
into a number of useful derivative compounds or to
be the basic material for the manufacture of other
compounds.
Some eugenol derivatives that have been carried
out are alkylation, addition, isomerization,
acetylation, esterification, polymerization, monoeter
cyclization and so on (Suryanto, 2008). Perangin-
angin (2019) have synthesized of 4-alil-6-
(hidroxymethyl)-2-methoxy phenol compounds from
eugenol through Mannich reaction followed
methylation with methyl iodide and substitution
using NaOH.
Eugenol is a class of phenylpropanoid chemical
compounds that have the potential for local
anesthetics that have been used medically by
dentists. There are phenol functional groups that
have antioxidant, anti-inflammatory, anti-allergic,
antithrombotic, antimicrobial and antineoplastic
activities (Soekardjo, 2000).
This ability is obtained from the lipophilic nature
of eugenol which can cause bacterial cell
membranes to undergo adhesion which causes
inhibited bacterial respiration. This will cause
disruption of ion transport in cells so that bacteria
experience death. In addition, phenol groups
contained in eugenol when attached to bacterial cells
will make bacteria undergo lysis, then die (Kumala,
2008).
Karanov et al. (1995) have synthesized eugenol
derivatives using formaldehyde and various types of
amines through the Mannich reaction. The new
compound formed is 2-methoxy-4- (2-propenyl) -6-
phenol-substituted aminomethyl derivative at
position 6 of eugenol which is known to have
activity as a plant growth regulator and pesticide.
Perangin-angin, S. and Chairi, S.
Synthesis of 6-Alyl-8-Methoxy-3-Propyl-1,3-Benzoxazine and 4-Alyl-6-(Dimetilamino)Methyl-2-Methoxy Phenol from Eugenol through Mannich Reaction and Antibacterial Activity Test.
DOI: 10.5220/0010205300002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 481-489
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
481

One example of a compound that has been
synthesized is 4-allyl-6- (dimethylamino) methyl-2-
methoxyphenol. The compound 4-allyl-6-
(dimethylamino) methyl-2-methoxyphenol can be
synthesized by reacting eugenol, dimethylamine, and
formaldehyde through the Mannich reaction.
Mannich reaction is a condensation reaction of
ammonia or primary amine or secondary amine and
formaldehyde with compounds containing acidic H
atoms bound to C or N atoms. In the Mannich
reaction, aldehyde condensation with ammonia or
primary amines or secondary amines will form the
Schiff base as an intermediate product.
The final product of the Mannich reaction is the
β-amino-carbonyl compound or the Mannich base
(Pine et al., 1988). Rudyanto et al. (2014)
synthesized benzoxazine and aminomethyl
compounds from eugenol and studied their
biological activity. Eugenol is reacted with
formaldehyde and methylamine following the
Mannich reaction. The benzoxazine compounds
obtained are then hydrolyzed to produce
aminomethyl derivatives. Furthermore, the
benzoxazine and aminomethyl compounds obtained
were tested for their biological activity using the
Brine Shrimp Lethality Test (BSLT), which is
testing the toxicity of a compound against Artemia
salina larvae.
Antibacterial activity test can be done by
diffusion and dilution methods. Disc diffusion test or
disk diffusion test is done by measuring the diameter
of the clear zone (clear zone) which is an indication
of the inhibitory response of bacterial growth by an
antibacterial compound in the extract. Requirements
for the number of bacteria for sensitivity test are
105-108 CFU / mL (Hermawan, 2007).
The diffusion method can be done in 3 ways
namely the cylinder method, the hole method and
the paper disc method. The hole method is to make a
hole in a solid agar that has been inoculated with
bacteria. The number and location of the holes are
adjusted to the purpose of the study, then the holes
are injected with the extract to be tested. After
incubation, bacterial growth was observed to see the
presence or absence of barriers around the hole
(Kusmayati, 2007).
Based on the above background, researchers are
interested in synthesizing benzoxazine and
aminomethyl compounds from eugenol through the
Mannich reaction using primary amines and
secondary amines to test their antibacterial activity.
2 MATERIALS AND METHOD
2.1 Synthesis of 6-allyl-8-methoxy-3-
propyl-1,3-benzoxazine
Compounds
Into the 100 mL three-neck flask with magnetic
stirrer included 28 mL ethanol and 4.8 g (0.03 mol)
eugenol were added. After dissolving, 37%
formaldehyde was added as much as 4 g (0.05 mol)
and 3.6 g (0.06 mol) propylamine followed by reflux
at 78
o
C for 6 hours. The mixture is cooled and
stirred with a magnetic stirrer for 24 hr.
Furthermore, the excess ethanol is evaporated with a
rotary evaporator. The results obtained were
analyzed with FT-IR and GC-MS
spectrophotometers.
2.2 Synthesis of 4-allyl-6-
(dimethylamino) methyl 2-methoxy
phenol Compound
Into the 100 mL three-neck flask with magnetic
stirrer included 28 mL ethanol and 4.8 g (0.03 mol)
eugenol were added. After dissolving, 37%
formaldehyde was added as much as 3.8 g (0.04
mol) and 5.6 g (0.05 mol) dimethylamine 40%
followed by reflux at 78
o
C for 90 minutes. The
mixture is cooled and stirred with a magnetic stirrer
for 24 hours. Furthermore, the excess ethanol is
evaporated with a rotary evaporator. The results
obtained were analyzed with FT-IR and GC-MS
spectrophotometers.
2.3 Preparation of Nutrient Agar Slant
About 7 g of NA was dissolved with 250 mL of
distillate water and sterilized in an autoclave at
121
o
C for 15 minutes.
2.4 Preparation of Medium Agar Slant
and Bacterial Culture Stock
The NA slant was prepared by adding 3 mL of NA
into test tube and placed it in the rack. Tilt the rack
onto solid surface so that the medium is slanted.
Allow the medium to harden in this position. The
culture was obtained from stock and taken with an
osse. This culture was incubated at 35
o
C for 18-24 h.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
482
2.5 Preparation of Mueller Hinton
Agar (MHA) Medium
Medium powder (19 g) was weighed into
erlenmeyer and dissolved with 500 mL of distillate
water sterilized in an autoclave at 121
o
C for 15
minutes.
2.6 Preparation of Bacterial Inoculum
Nutrient broth (3.25 g) was dissolved with 250 mL
of distillate water and sterilized in an autoclave at
121
o
C for 15 minutes. Furthermore, microbial
bacterial colony was taken from culture stock using
a sterilized osse. The culture was suspended into 10
mL of sterilized nutrient broth in the test tube and
incubated at 35
o
C for 3 h. The optical density of
bacterial was determined using spectrophotometer
UV-Vis at 580-600 nm.
2.7 Evaluation of Antibacterial Activity
The antibacterial activity of quaternary ammonium
salt was obtained by diffusion method. Paper disk (Ǿ
6 mm) had been soaked in various concentration of
quaternary ammonium salt (10, 20, and 30%). This
paper disk then placed on the agar medium that has
been cultured with E. coli and S. mutans. The
inhibition zone was measured using calliper (mm).
3 RESULTS AND DISCUSSION
3.1 Synthesis of 6-allyl-8-methoxy-3-
propyl-1,3-benzoxazine
Compounds
Eugenol used in this study is eugenol p.a E'Merck
with a purity level of ≥99%. The 6-allyl-8-methoxy-
3-propyl-1,3-benzoxazine compound obtained in the
form of a mixture of 6.116 grams (82.54%), in the
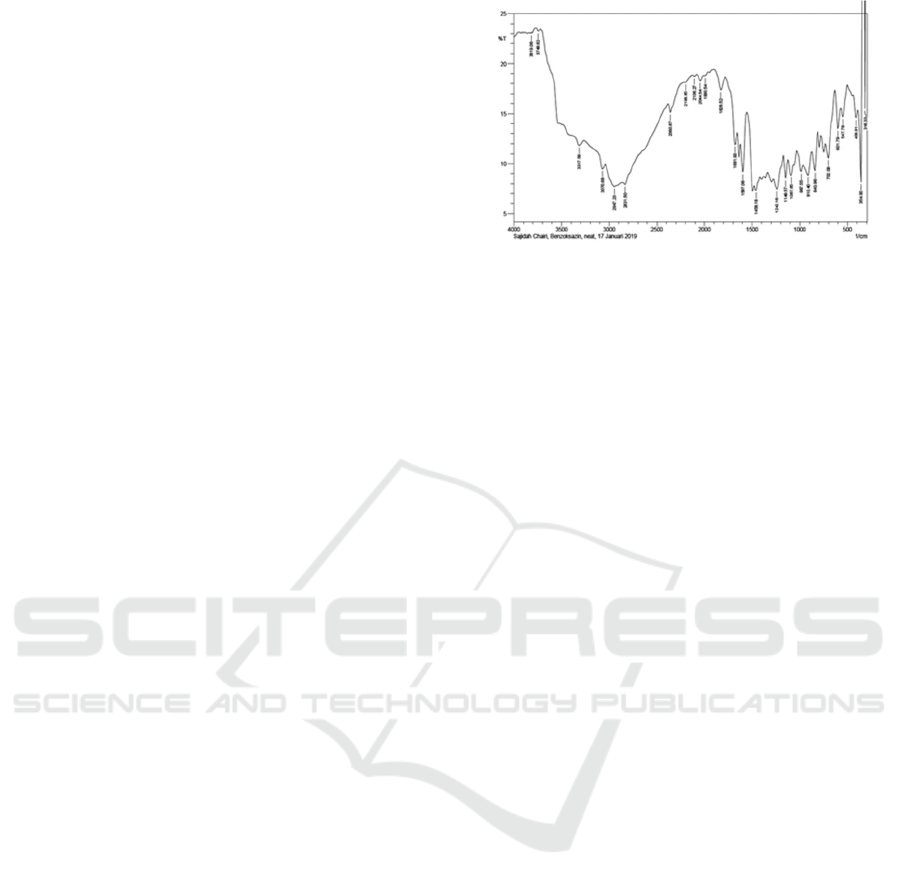
form of a blackish-brown liquid.
FT-IR spectroscopic data of 6-allyl-8-methoxy-
3-propyl-1,3-benzoxazine compound gives a
spectrum with vibrational peaks in the region of
wave number 3070.68 cm-1; 2947.23 cm-1; 2831.50
cm-1; 1597.06 cm-1; 1458.18 cm-1; 1242.16 cm-1;
1149.57 cm-1; 987.55 cm-1. The results of FT-IR
analysis of 6-allyl-8-methoxy-3-propyl-1,3-
benzoxazine compounds can be seen in Figure 1.
Figure 1: FT-IR of 6-allyl-8-methoxy-3-propyl-1,3-
benzoxazine compound.
The spectrum shown from FT-IR data supports
that the compound 6-ally-8-methoxy-3-propyl-1,3-
benzoxazine has a CN bond originating from the
benzoxazine group with the appearance of CN
stretching vibrations at wave number 1242,16 cm-1.
The absorption peak at wave number 3070.68 cm-1
shows the range of CH sp2, and the area of 2947.23
cm-1 and 2831.50 cm-1 shows the range of CH sp3
of alkyl which is reinforced by the peak at 1458.18
cm-1 for the group methylene (―CH2―) and C = C
aromatic are shown at wave number 1597.06 cm-1.
The vinyl group is shown at 987.55 cm-1. The peak
at 1149.57 cm-1 shows the stretch C-O-C of the
ether.
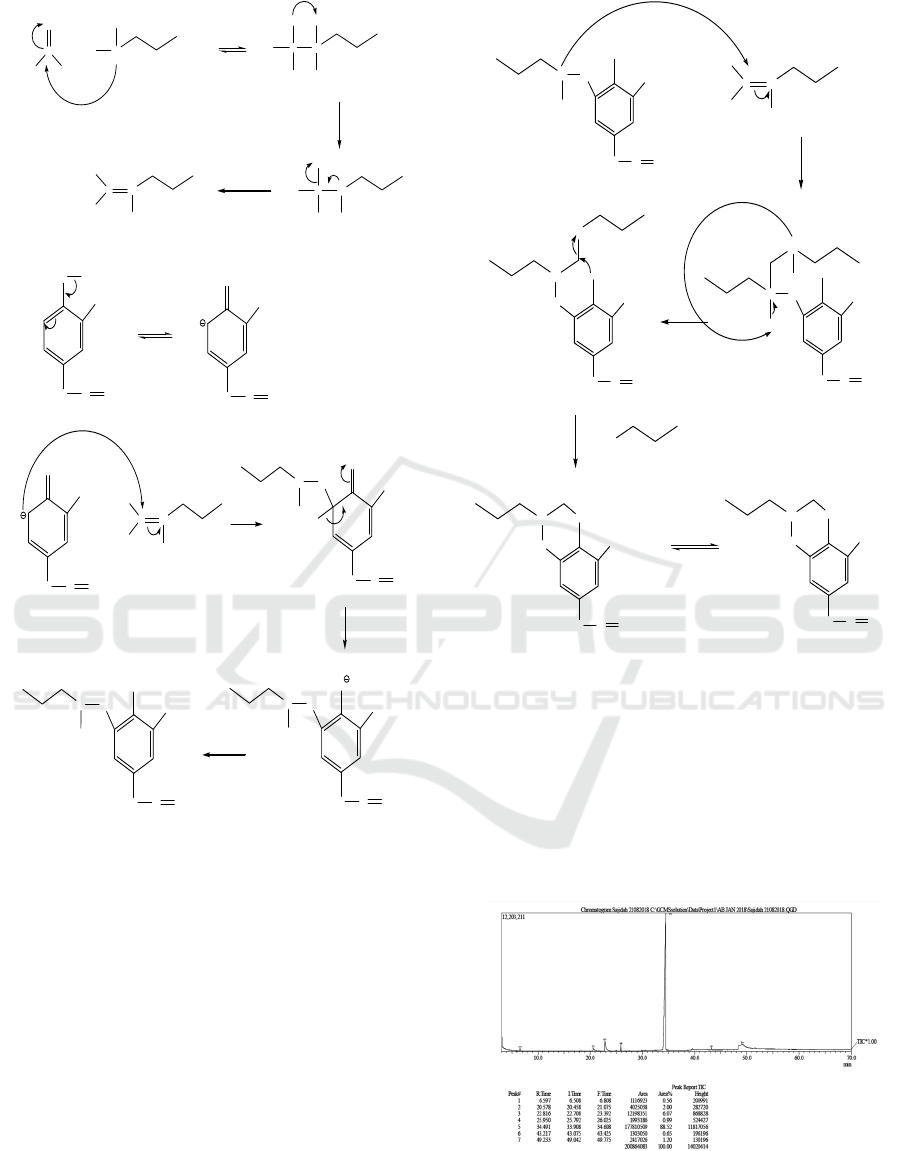
The 6-allyl-8-methoxy-3-propyl-1,3-benzoxazine
compound is obtained from eugenol through the
Mannich reaction, where eugenol is reacted with
iminium ions which were previously formed through
the reaction between formaldehyde and propylamine.
In the Mannich reaction of eugenol, the active
hydrogen from eugenol is replaced by the
propylaminomethyl group as an iminium ion. Then
the active nitrogen from the propylaminomethyl
group attacks the aluminum ion again to form an
oxazine ring by releasing propylamine. The Mannich
reaction was carried out under reflux conditions at a
temperature of 78 ° C for 6 hours using ethanol
solvent. The results of the eugenol, formaldehyde and
propylamine reactions produce 6-allyl-8-methoxy-3-
propyl-1,3-benzoxazine derivatives.
The mechanism of the 6-allyl-8-methoxy-3-
propyl-1,3-benzoxazine reaction can be seen in
Figure 2.
Synthesis of 6-Alyl-8-Methoxy-3-Propyl-1,3-Benzoxazine and 4-Alyl-6-(Dimetilamino)Methyl-2-Methoxy Phenol from Eugenol through
Mannich Reaction and Antibacterial Activity Test
483
. .
C
O
H
. .
:
+
N
H
H
N
H
C
O
-
H
H H
N
C
OH
H
H H
. .
-OH
N
C
H
H
H
+
Formaldehid
Propilamin
Transfer proton
Ion iminium
CN
H
H
+
+
O
H
2
C C
H
CH
2
OCH
3
H
CH
2
N
OH
H
2
C C
H
CH
2
OCH
3
CH
2
N
O
H
2
C C
H
CH
2
OCH
3
H
O
H
2
C C
H
CH
2
OCH
3
O
H
2
C C
H
CH
2
OCH
3
O
H
2
C C
H
CH
2
OCH
3
CH
2
N
+ H
+
-H
+
. .
. .
. .
H
H
H
H
H
2
C C
H
CH
2
OCH
3
CH
2
N
OH
H
N
C
H
H
H
+
+
H
2
C C
H
CH
2
OCH
3
CH
2
N
OH
H
N
+
H
2
C C
H
CH
2
OCH
3
H
2
C
N
OH
H
2
N
H
2
C C
H
CH
2
OCH
3
H
2
C
N
OH
+
- H
+
H
2
C C
H
CH
2
OCH
3
H
2
C
N
O
6-alil-8-metoksi-3-propil-1,3-benzoksazin
H
2
N
..
H
+
Figure 2: Mechanism of 6-allyl-8-methoxy-3-propyl-1,3-
benzoxazine reaction.
The results of the analysis with GC-MS on6-
allyl-8-methoxy-3-propyl-1,3-benzoxazine
compounds obtained from the synthesis showed a
peak retention time of 34,491 minutes with a purity
of 88.52%. Mass chromatograms of compounds
synthesized by GC-MS can be seen in Figure 3.
Figure 3: GC-MS spectra of identified compounds.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
484
The spectrum of compound 6-allyl-8-methoxy-3-
propyl-1,3-benzoxazine obtained is shown in Figure
4.
Figure 4: Peak detection of identified compounds.
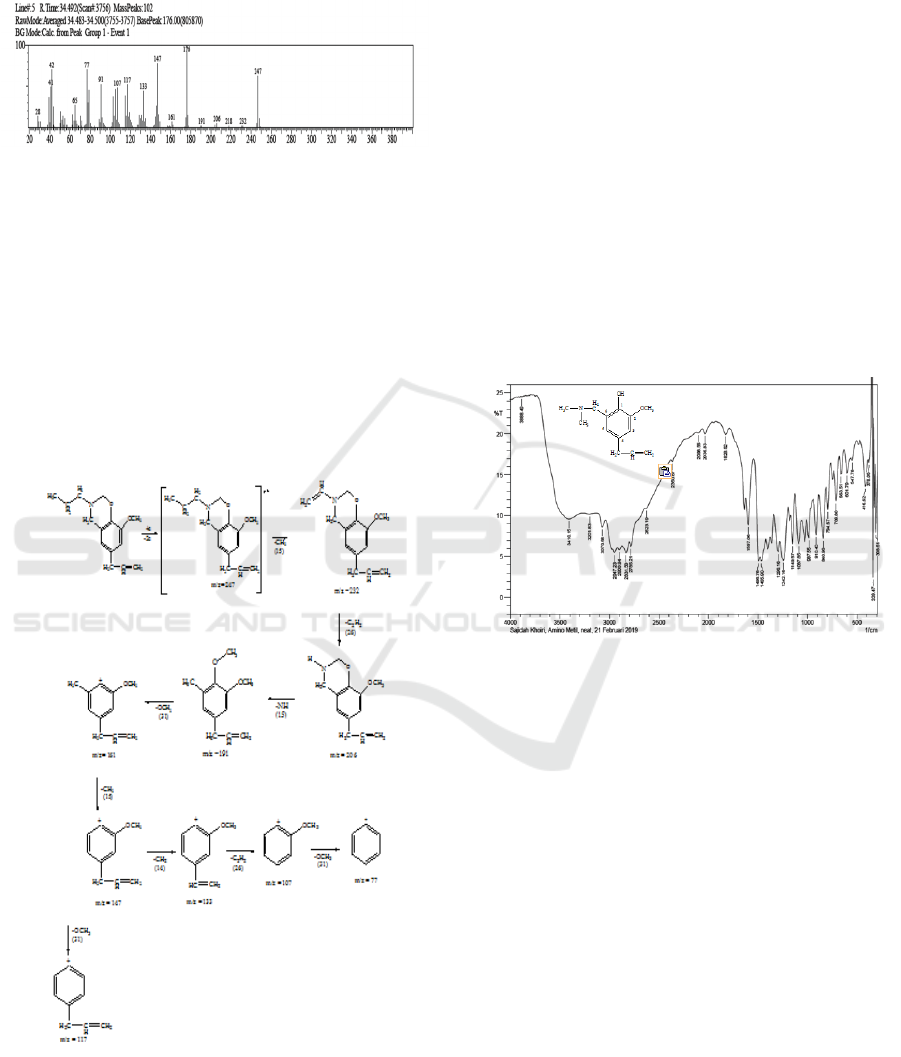
The peak with a retention time of 34.491 minutes
is a compound with the molecular formula
C
15
H
21
NO
2
with a relative molecular mass of 247 g /
mol. Spectrum data show molecular ion peaks at m /
e 247 followed by fragmentation peaks at m / e 232,
218, 206, 191, 176, 161, 147, 133, 117, 107, 91, 77,
65, 42, 41, and 28 where this value corresponds to
the relative molecular weight (Mr) of the
synthesized 6-ally-8-methoxy-3-propyl-1,3-
benzoxazine compound. Fragmentation patterns can
be seen in Figure 5.
Figure 5: Fragmentation pattern of synthesized compound.
3.2 Synthesis of 4-allyl-6-
(dimethylamino) compound
methyl-2-methoxy phenol
Eugenol used in this study is eugenol p.a E'Merck
with a purity level of ≥99%. Compound 4-allyl-6-
(dimethylamino) methyl-2-methoxy phenol obtained
in the form of a mixture of as much as 5.728 grams
(86.39%), in the form of a blackish brown liquid.
FT-IR spectroscopy data of 4-allyl-6-
(dimethylamino) methyl-2-methoxy phenol
compound gives a spectrum with vibrational peaks
in the region of wave number 3410.15 cm-1;
3070.68 cm-1; 2947.23 cm-1; 2900.94 cm-1;
2831.50 cm-1; 1597.06 cm-1; 1465.90 cm-1;
1242.16 cm-1; 1149.57 cm-1; 987.55 cm-1. The
results of FT-IR analysis of 4-allyl-6-
(dimethylamino) methyl-2-methoxy phenol
compounds can be seen in Figure 6.
Figure 6: FT-IR of 4-allyl-6- (dimethylamino) methyl-2-
methoxy phenol compound.
The spectrum shown from FT-IR data supports
that the 4-allyl-6- (dimethylamino) compound
methyl-2-methoxy phenol formed has a CN bond
originating from the dimethylaminomethyl group
with the emergence of CN stretching vibrations at
the wave number 1242.16 cm- 1 The absorption
peak at wave number 3410.15 cm-1 shows the O-H
vibrations. The absorption peak at wave number
3070.68 cm-1 shows the range of C-H sp2 and in the
area of 2947.23 cm-1; 2900.94 cm-1 and 2831.50
cm-1 shows the CH sp3 range of alkyl reinforced
with a peak at 1465.90 cm-1 for the methylene
group (―CH2―) and C = C aromatic shown at
wave number 1597.06 cm-1. The vinyl group is
shown at 987.55 cm-1. The tape at 1149.57 cm-1
shows the stretch C-O-C of the ether.
The 4-allyl-6- (dimethylamino) methyl-2-
methoxy phenol compound is obtained from eugenol
through the Mannich reaction, where eugenol is
reacted with iminium ions which were previously
Synthesis of 6-Alyl-8-Methoxy-3-Propyl-1,3-Benzoxazine and 4-Alyl-6-(Dimetilamino)Methyl-2-Methoxy Phenol from Eugenol through
Mannich Reaction and Antibacterial Activity Test
485
formed through the reaction between formaldehyde
and dimethylamine. In the Mannich reaction, the
active hydrogen from eugenol is replaced by the
dimethylaminomethyl group. The Mannich reaction
was carried out under reflux conditions at a
temperature of 78°C for 90 minutes using ethanol as
a solvent. The results of the eugenol, formaldehyde
and dimethlamine reactions produce the 4-allyl-6-
(dimethylamino) methyl-2-methoxy phenol
derivative.
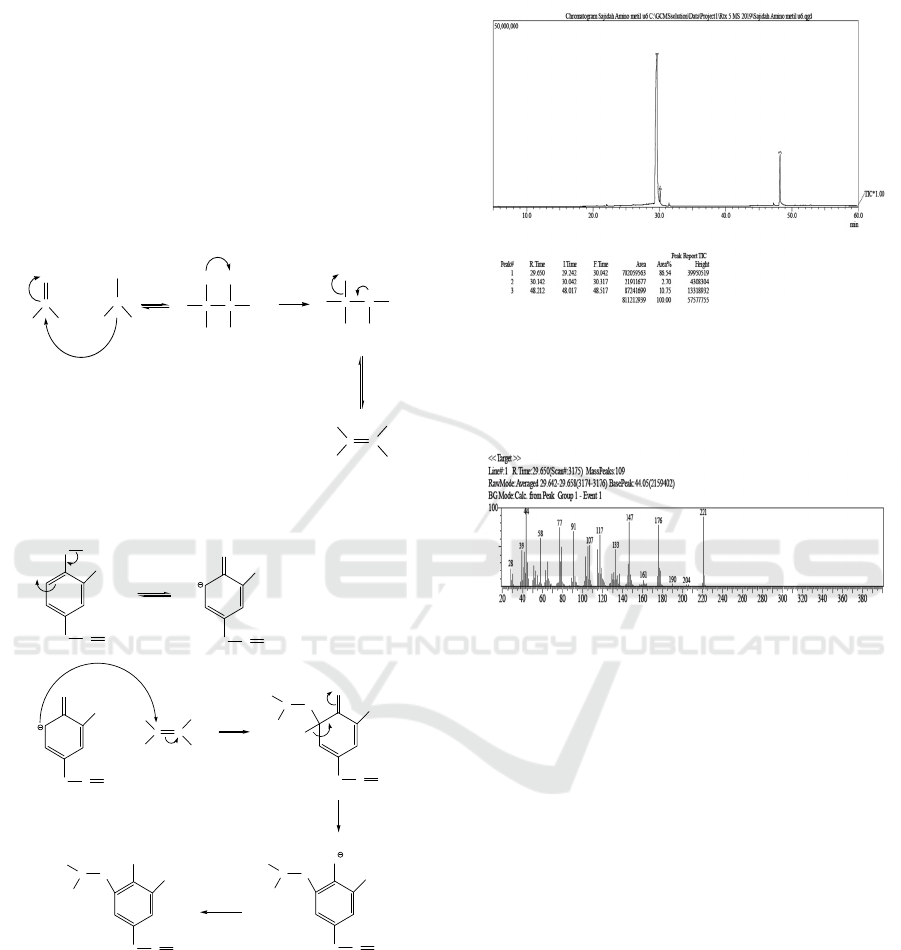
The reaction mechanism of the 4-allyl-6-
(dimethylamino) methyl-2-methoxy phenol
compound can be seen in Figure 7.
N
CH
3
H
+
H
3
C
HC
O
-
H
N
H
+
CH
3
CH
3
CN
Dimetilamin
. .
H CH
3
CH
3
H
+
Transfer proton
Ion iminium
HC
OH
H
N
CH
3
CH
3
-OH
-
C
O
H
Formaldehid
. .
:
. .
CN
H CH
3
CH
3
H
+
+
O
H
2
C C
H
CH
2
OCH
3
H
CH
2
N
H
3
C
H
3
C
OH
H
2
C C
H
CH
2
OCH
3
CH
2
N
H
3
C
H
3
C
4-alil-6-(dimetilamino)metil-2-metoksi fenol
O
H
2
C C
H
CH
2
OCH
3
H
O
H
2
C C
H
CH
2
OCH
3
O
H
2
C C
H
CH
2
OCH
3
O
H
2
C C
H
CH
2
OCH
3
CH
2
N
H
3
C
H
3
C
+ H
+
-H
+
. .
. .
. .
Figure 7: Reaction mechanism of the 4-allyl-6-
(dimethylamino) methyl-2-methoxy phenol.
Results of analysis with GC-MS on 4-allyl-6-
(dimethylamnino) methyl-2-methoxy phenol
compounds obtained from the synthesis showed a
peak retention time of 29,650 minutes with a purity
of 86.54%. Mass chromatograms of compounds
synthesized by GC-MS are shown in Figure 3.8.
Figure 8: GC-MS spectra of identified compounds.
The spectrum of the compound 4-allyl-6-
(dimethylamino) methyl-2-methoxy phenol obtained
was shown in Figure 9.
Figure 9: Peak detection of identified compounds.
The peak with a retention time of 29,650 minutes
is a compound with the molecular formula
C
13
H
19
NO
2
with a relative molecular mass of 221 g /
mol. Spectrum data show the peaks of molecular
ions at m / e 221 followed by fragmentation peaks at
m / e 204, 190, 176, 161, 147, 133, 117, 107, 91, 77,
58, 44, 39, and 28, where this value corresponds to
the relative molecular weight (Mr) of the 4-allyl-6-
(dimethylamino) compound synthesized methyl-2-
methoxy phenol. Fragmentation patterns can be seen
in Figure 10.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
486
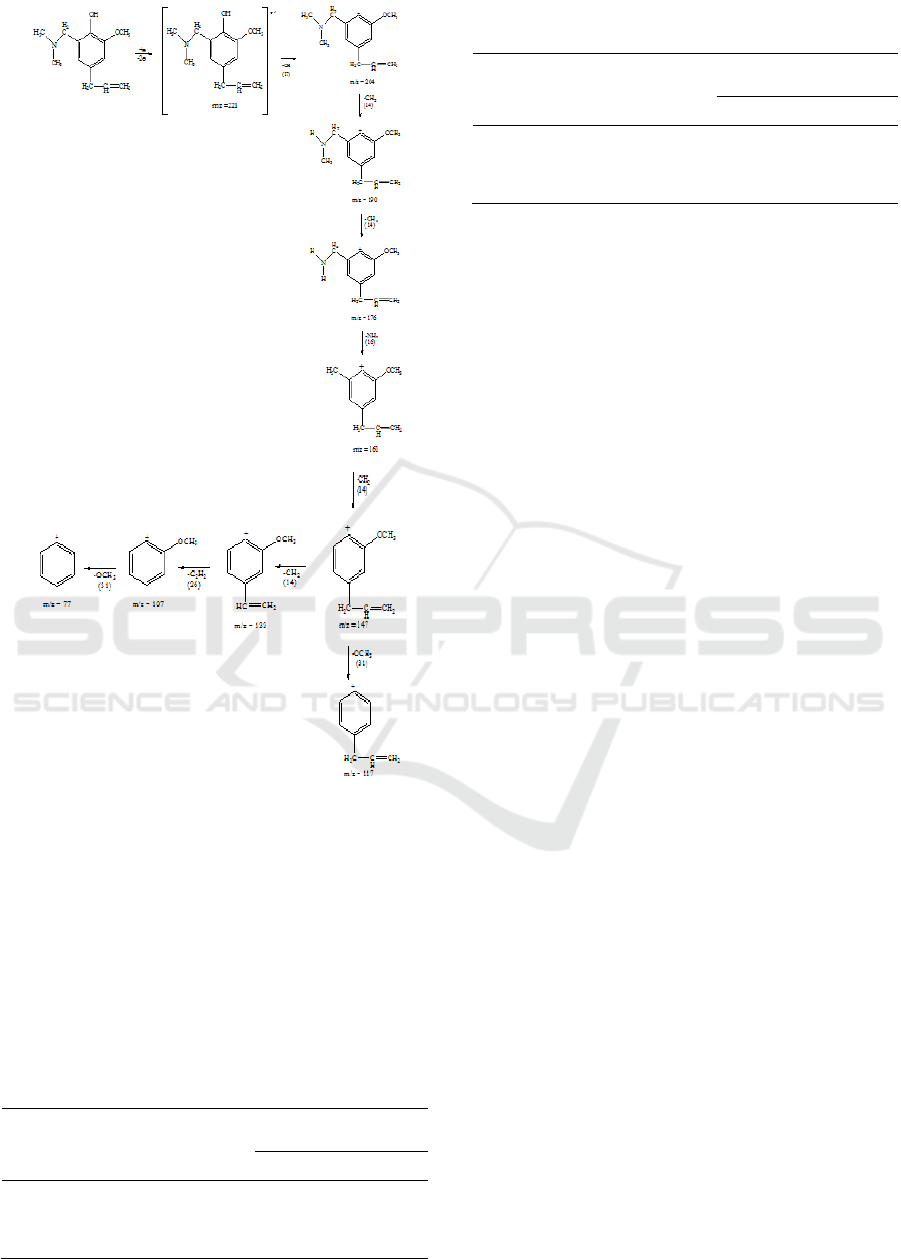
Figure 10: Fragmentation pattern of 4-allyl-6-
(dimethylamino) compound.
3.3 Antibacterial Activity Test
Antibacterial activity test of 6-allyl-8-methoxy-3-
propyl-1,3-benzoxazine compound and 4-allyl-6-
(dimethylamino) methyl-2-methoxy phenol
compound from the synthesis results in the form of
mixture using Streptococcus mutans and Eschericia
coli can be seen in Table 1 and 2.
Table 1: Tests for antibacterial activity against 6-allyl-8-
methoxy-3-propyl-1,3-benzoxazine compounds.
Treatment
Disc Diameter
(mm)
Clear Zone Diameter
(
mm
)
S. mutans E. coli
10%
6 18.5 21
20%
6 33 21
30%
6 33 14
Table 2: Tests for antibacterial activity against 4-allyl-6-
(dimethylamino) methyl-2-methoxy phenol compounds.
Treatment
Disc Diameter
(mm)
Clear Zone Diameter
(
mm
)
S. mutans E. coli
10% 6 12 18
20% 6 19 16
30% 6 17 14
Data on antibacterial activity test results showed
that there were antibacterial activities for both
Streptococcus mutans and Escherichia coli bacteria
in 6-allyl-8-methoxy-3-propyl-1,3-benzoxazine and
4-allyl-6- (dimethylamino) methyl-compound 2-
methoxy phenol. This is because the 6-allyl-8-
methoxy-3-propyl-1,3-benzoxazine compound and
the 4-allyl-6- (dimethylamino) methyl-2-methoxy
phenol compound have a nitrogenous base group.
This base group will react with amino acids that
make up the cell wall and bacterial DNA which are
the main constituents of the cell nucleus. This
reaction results in changes in the structure and
composition of amino acids that cause changes in
genetic balance so that the bacterial DNA will be
damaged. Cell damage to bacteria will eventually
make the bacteria unable to metabolize so that it will
also undergo lysis. Thus, the bacteria will become
inactive and destroyed (Gunawan, 2008).
In the antibacterial activity test of 6-allyl-8-
methoxy-3-propyl-1,3-benzoxazine compound
against Streptococcus mutans, the antibacterial
activity is stronger than eugenol. This is shown by
the highest clear zone diameter at concentrations of
20% and 30%, which is 33 mm while in eugenol the
highest clear zone diameter is 18 mm at a
concentration of 10%.
In the antibacterial activity test of 6-allyl-8-
methoxy-3-propyl-1,3-benzoxazine compound
against Escherichia coli, the highest clear zone
diameter at concentrations of 10% and 20% is 21
mm and has decreased at a concentration of 30%
that is equal to 14 mm. In the antibacterial activity
test of 4-allyl-6- (dimethilamino) methyl-2-methoxy
phenol compound against Streptococcus mutans the
highest clear zone diameter was obtained at a
concentration of 20% at 19 mm and decreased at a
concentration of 30% at 17 mm, whereas, against
Escherichia coli the highest clear zone diameter at a
concentration of 10% is 18 mm and has decreased at
20% and 30% that is 16 mm and 14 mm.
Based on the test results of antibacterial activity
against bacteria Streptococcus mutans and
Escherichia coli obtained inhibitory zone diameters
Synthesis of 6-Alyl-8-Methoxy-3-Propyl-1,3-Benzoxazine and 4-Alyl-6-(Dimetilamino)Methyl-2-Methoxy Phenol from Eugenol through
Mannich Reaction and Antibacterial Activity Test
487

that have fluctuated and not proportional to the
concentration of the compound.
Elifah (2010) suggested that the diameter of the
inhibition zone does not always increase in
proportion to the increase in antibacterial
concentration. This can occur because of differences
in the speed of diffusion of antibacterial compounds
on agar media. Different types and concentrations of
antibacterial compounds give different inhibitory
zone diameters for a certain period of time.
Irregularity in the diameter of the zone of inhibition
of the growth of test bacteria is at the time of
unequal disk drying. Therefore, it causes a inhibitory
zone at the highest concentration to decrease. Disk
which has long drying time, when it is placed on top
of the bacterial hatching media, the area of the
inhibitory zone is small, this zone is formed from
extracts diffused from the disk to the agar media. On
disks with only a short drying time, when placed on
top of the bacterial hatchery media, the extract
which is still attached immediately spreads around
the disk and quickly diffuses to the media so as to
form a larger inhibitory zone.
Sinarsih (2016) suggests that the presence of
unstable antibacterial performance at high
concentrations is likely due to compounds in general
having a limited ability in bioactivity. So that at
increasing concentrations certain compounds do not
provide a significant increase in response or not
significantly different.
The strength of antibacterial activity can be seen
from the inhibitory zone formed. According to
(Aleksandra et al., 2017) said that antibacterial
activity was classified to be 3 groups. There were
strong that produced inhibition zone diameter at 8
mm, medium activity that produced inhibition zone
at 7-8 mm, while weak activity that produced
inhibition zone diameter less than 7 mm. Thus 6-
allyl-8-methoxy-3-propyl-1,3-benzoxazine
compound and 4-allyl-6- (dimethylamino) methyl-2-
methoxy phenol compounds have relatively strong
antibacterial activity.
4 CONCLUSION
Synthesis of 6-allyl-8-methoxy-3-propyl-1,3-
benzoxazine compound obtained from eugenol
through the Mannich reaction, where eugenol is
reacted with iminium ions which were previously
formed through the reaction between formaldehyde
and propylamine. The results obtained were 6.116
grams (82.54%) compound of 6-allyl-8-methoxy-3-
propyl-1,3-benzoxazine. The formation of 6-allyl-8-
methoxy-3-propyl-1,3-benzoxazine compound is
characterized by the appearance of C-N stretching
vibrations at wave number 1242.16 cm-1
Synthesis of 4-allyl-6- (dimethylamino) methyl-
2-methoxy phenol compound obtained from eugenol
through the Mannich reaction, where eugenol is
reacted with iminium ions which were previously
formed through the reaction between formaldehyde
and dimethylamine. The results obtained were 5,728
grams (86.39%) of the 4-allyl-6- (dimethylamino)
compound methyl-2-methoxy phenol. The formation
of 4-allyl-6- (dimethylamino) compound methyl-2-
methoxy phenol is characterized by the appearance
of C-N stretching vibrations at wave number
1242.16 cm-1 and O-H vibrations at wave number
3410.15 cm-1
The 6-allyl-8-methoxy-3-propyl-1,3-benzoxazine
compound and the 4-allyl-6- (dimethylamino)
methyl-2-methoxy phenol compound exhibit
antibacterial activity that is classified as strong
against S. mutans and E bacteria .coli. This is
indicated by the diameter of the clear zone produced
which is more than 8 mm so that both compounds act
as antibacterials that are classified as strong.
ACKNOWLEDGEMENTS
Author would like to thank to organic chemistry
laboratory faculty of mathematics and natural
sciences University of Sumatera Utara.
REFERENCES
Aleksandra, O.B., N, D.Z., Rzepkowska, A., Ko,D., 2017.
Comparison of Antibacterial Activity of Lactobacillus
plantarum Strains Isolated from Two Different Kinds
of Regional Cheeses from Poland : Oscypek and
Korycinski Cheese.
Gunawan, I.W.A. 2008. Isolasi dan Identifikasi Senyawa
Terpenoid yang Aktif Antibakteri pada Herba Meniran
(Phyllanthus Niruri Linn). Jurnal Kimia2 (1): 31-39.
Hermawan. A., 2007. Pengaruh Ekstrak Daun Sirih (Piper
betle L.) terhadap Pertumbuhan Staphylococcus
aureus dan Escherichia coli Dengan Metode Difusi
Disk, Artikel Ilmiah, Fakultas Kedokteran Hewan,
Universitas Airlangga Surabaya.
Karanov, E., L. Iliev, V. Alexieva, G. Ts. Georgiev, N. T.
Thang, and L. Natova, 1995, Synthesis and
Plant Growth Regulating Activity of some Novel 2-
Methoxy-4-(1-Or2 Propenyl)-6-Substituted Phenol,
Journal Plant Physiology,21(4),39- 47.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
488

Kumala, Shirly, dan Dian Indriani. 2008. Efek Antibakteri
Ekstrak Etanol Daun Cengkeh (Eugenia aromaticum
L). Jurnal Farmasi Indonesia , 4(2):82-86.
Kusmayati, Agustini., 2007. Uji Aktivitas Senyawa
Antibakteri dari Microalga (Prorpyridium cruentum).
Jurnal Biologi Universitas Atmajaya. Yogyakarta.
Perangin-angin, S., 2019. Synthesis of 4-Alil-6-
(Hidroxymethyl)-2-Methoxy Phenol Compounds from
Eugenol Through Mannich Reaction Followed
Methylation with Methyl Iodide and Substitution
Using NaOH. JCNaR.01,75-85
Pine, S. H., J. B. Hendrikson, D. J. Cram, dan G. S.
Hammond, 1988, Kimia Organik, edisi IV,
terjemahan R. Joedodibroto dan S. W. P.
Hadiwidjoyo, ITB, Bandung, 152-153, 325-326
Rudyanto, M., Ekowati, J., Widiandani, T., Honda, T.
Synthesis and brine shrimp lethality test of some
benzoxazine and aminomethyl derivatives of eugenol
International Journal of Pharmacy and Phamaceutical
Sciences.2014; 6(2): 465-467
Sinarsih, N. K., Rita, W. S., Puspawati, N. M ., 2016. Uji
Efektifitas Ekstrak Daun Trembesi (Samanea saman
(jacq.) Merr) Sebagai Antibakteri Escherichia coli dan
Staphylococcus aureus. Journal of Applied Chemistry;
4(2).
Soekardjo, B. dan Sondakh, R. 2000. Hubungan Struktur-
Aktivitas Obat Antiinfeksi, dalam :Kimia Medisinal,
Siswandono dan B. Soekardjo (eds.), ed. 2,
jil.2,Airlangga University Press, Surabaya, 21
Synthesis of 6-Alyl-8-Methoxy-3-Propyl-1,3-Benzoxazine and 4-Alyl-6-(Dimetilamino)Methyl-2-Methoxy Phenol from Eugenol through
Mannich Reaction and Antibacterial Activity Test
489
