
Synthesis and Characterization of Strontium Hexa Ferrite (SrFe
12
O
19
)
Powder by using Powder Metallurgy Method
Ramlan
1*
, Dedi Setiabudidaya
1
, A. Aminuddin Bama
1
, Akmal Johan
1
, Muljadi
2
and P. Sardjono
3
1
Department of Physics, University of Sriwijaya, Palembang, South Sumatera, Indonesia
2
Research Center for Physics, Indonesian Institute of Sciences, PuspiptekSerpong, South Tangerang, Indonesia
3
Mechanical Engineering Department, University of Pamulang, South Tangerang, Indonesia
Keywords: Sr-ferrite, Permanent Magnet, Powder Metallurgy, Hysteresis Curve, Crystal Structure, Coercivity.
Abstract: The Strontium hexa ferrite with formula SrFe
12
O
19
or SrO 6Fe
2
O
3
is permanant magnet materials , it has
high magnetic properties, high curie temperature (350
o
C) and good corrosion resistance. This research was
done in preparation of Strontium hexa ferrite by using raw materials : hematite (Fe
2
O
3
) and SrCO
3
at mole
ratio SrO:Fe
2
O
3
= 1 :6. The both raw materials were weighed and mixed by using HEM for 4 hours and
aquadest as milling media. After that, the sample was dried at 110
o
C for 4 hours by using drying oven. The
dried sample was analyzed by using DTA/TG to know the calcination temperature. According DTA/TG
curve, there are 3 peaks endothermic at 730
o
C, 820
o
C and 990
o
C. After that , the sample was calcined at
temperature 900
o
C and 1000
o
C for 2 hours. The calcined samples were analyzedcrystall structure by using
XRD, measured magnetic properties by using VSM. According XRD results show that the first formation of
SrFe
12
O
19
phase at temperature 900
o
C, With the increasing of calcination temperature can increasing of
SrFe
12
O
19
phase. The VSM results show that it is obtained a wide hysteresis curve with highest coercivity
value 3000Oe.
1 INTRODUCTION
There are some of types materials as permanent
magnet such as materials based on ferrite, metals
alloy (SmCo and AlNiCo) and rare earth metals
alloy (NdFeB), each of these materials exhibits
different set of properties (Sebayang et al., 2011).
Permanent magnets are widely used in various
fields, among others, in the automotive industry, as
components of divais energy (generators), sensor
industry, components in medical equipment and
others (Nowosielski et al., 2007). There are two
types of ferrite permanent magnet namely Sr ferrite
and Ba ferrite, which the magnetic properties of Sr
ferrite are slightly higher than Ba ferrite, for
example energy product of Sr ferrite is about 0.30
kJ/m
3
and about 27 for Ba ferrite (Slusarek &
Zakrzewski, 2012). Sr ferrite with formula SrFe
12
O
19
is one of type permanent magnet materials based on
ferrite, and it is called as ceramic magnet. The Sr
ferrite has hexagonal closed pack crystal structure
and this magnet has large magneto ainisotropic,
stable and good corrosion resitance also has high
curie temperature about 450
o
C (Nowosielski et al.,
2007; Slusarek & Zakrzewski, 2012). The magnetic
properties of permanent magnet based on ferrite is
still lower than other types of permanent magnet, but
the raw materials cost and manufacturing cost of
ferrite magnet are lower than other types of
permanent magnet, because the main raw materials
such as hematite (Fe
2
O
3
) are abundant in nature.
Among the class of permanent magnet materials the
ferrite magnet are very important due to its moderate
magnetic properties at lower cost. Therefore ferrite
magnet is still used until now and many of the
researches in this field are conducted to improve the
physical and magnetic properties (Mahmood & Abu-
Aljarayesh, 2016; Slusarek et al., 2013). The
reaction mechanism of SrFe
12
O
19
formation is
through the solid reaction mechanism and there are
two steps of forming reaction, as follow (Pullar,
2012):
SrO + Fe
2
O
3
======= SrO.Fe
2
O
3
(1)
SrO.Fe
2
O
3
+5Fe
2
O
3
=== SrO.6Fe
2
O
3
(2)
The first reaction step is an intermediate phase
formation reaction (SrO.Fe
2
O
3
) that takes place at
460
Ramlan, ., Setiabudidaya, D., Bama, A., Johan, A., Muljadi, . and Sardjono, P.
Synthesis and Characterization of Strontium Hexa Ferrite (SrFe12O19) Powder by using Powder Metallurgy Method.
DOI: 10.5220/0010204800002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 460-463
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
high temperatures, then continued with formation of
SrO.6Fe
2
O
3
or SrFe
12
O
19
at higher temperature.
Mechanism of formation reaction dependson
temperature , particle size and homogeneity of
mixing (Pullar, 2012). The high temperature plays
an important role in the formation of BaFe
12
O
19
phase. If the temperature reaction is too high, that
can lead to grain growth and can influence magnetic
properties (Pullar, 2012; Takahashi et al., 2012).
Ferrite magnetic particle powder can be prepared
through several techniques including through wet
chemical process (such as a cooprecipitation , sol gel
and Freeze drying) and through solid-solid mixing
which raw materials are in solid compound
(Ghobeiti-Hasab, 2014; Nowosielski et al., 2008).
The preparation of ferrite magnet using solid-solid
mixing is called also a powder metallurgy process
(Nowosielski et al., 2008). The powder metallurgy
process is a simple process and it is needed a simple
equipment, cheap raw materials. Some of parameters
such as impurity, particle size and homogeneity of
mixing give effect on physical and magnetic
properties also on crystal structure (Moosa, 2013;
Nowosielski et al., 2008). This research was
conducted to synthesis of Sr ferrite (SrFe
12
O
19
) using
powder metallurgy technique and the purpose of this
study was to determine the effect of combustion
temperature variations on changes in crystal
structure and magnetic properties.
2 EXPERIMENT WORKS
This research was done in preparation of Sr ferrite
with formula SrFe
12
O
19
by metallurgy method,
Hematite (Fe
2
O
3
E-Merck) and SrCO
3
(E-
merck) were used as raw materials and mole ratio
SrO:Fe
2
O
3
= 1 :6 is applied for synthesis of Sr ferrite
powder. The both raw materials were weighed and
wet milled by using High Energy Milling (HEM) for
4 hours and used aquadest as milling media. After
that, the sample was dried at 110
o
C for 4 hours by
using drying oven. The dried sample was analyzed
by using DTA/TG to know the calcination
temperature. According to DTA/TG curve, there are
3 peaks endothermic at 730
o
C, 820
o
C and 990
o
C.
After that , the sample was calcination at different
temperature such as : 900 and 1000
o
C for 2 hours.
The calcined samples were analyzedcrystall
structure by using XRD, measured magnetic
properties by using VSM.
3 RESULTS AND DISCUSSION
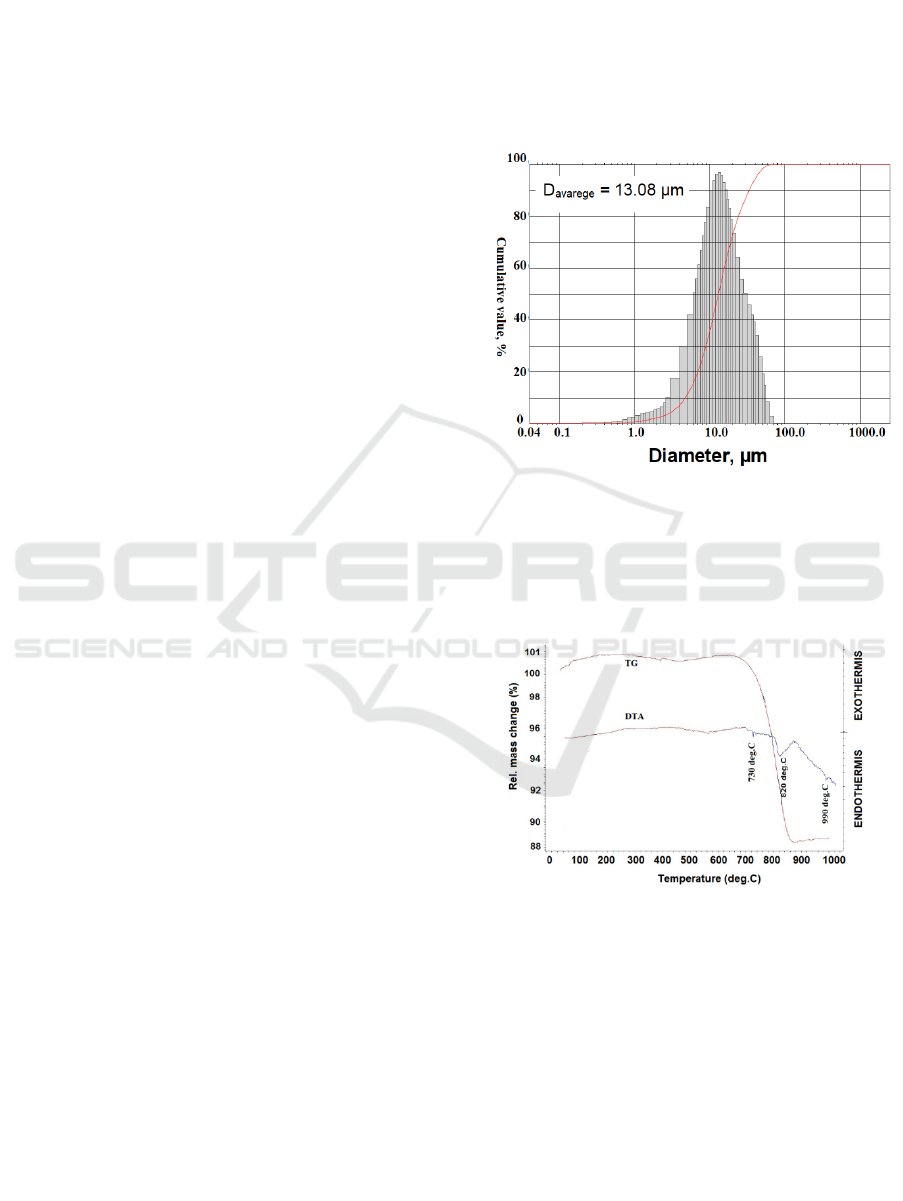
The mixed raw material after milling 4 hours using
HEM was measured particle size distribution and the
result is seen in Figure 1. Based on Figure 1, it can
be obtained that the average particle diameter is
determined at a cumulative point of 50 %, so that the
average particle diameter value is 13.08 µm.
Figure 1: Particle size distribution curve after milling 4
hours.
The mixed raw material was measured using
DTA/TG Analyser before calcination process and
the result is seen at Figure 2.
Figure 2: The DTA/TG curve of mixed raw material.
The mixed raw material consists of SrCO
3
and
Fe
2
O
3
, according to DTA/TG curve , it was found
that there are 3 peaks endothermic at temperature
730, 820 and 990
o
C. The reaction decomposition of
SrCO
3
was occurred at temperature 730 and 820
o
C
to form SrO, which it indicates with sharply
decreasing of mass from temperature 700
o
C to
880
o
C , then continued second reaction to form
SrFe
12
O
19
at temperature 990
0
C, where there are not
mass change. Based on the DTA/TG results that the
mixed raw material was conducted calcination using
Synthesis and Characterization of Strontium Hexa Ferrite (SrFe12O19) Powder by using Powder Metallurgy Method
461

electrical furnace at temperature 900
o
C and 1000
o
C.
The XRD results of calcined samples are shown at
Figure 3. The phase identification was done using
Match soft ware through matching experiment data
with reference data. The xrd result show that it is
found two peaks namely hematite (Fe
2
O
3
) phase and
SrFe
12
O
19
phase at sample after calcination at 900
o
C.
It is indicated that the starting of forming phase is at
temperature 900
o
C, but the reaction of SrF formation
at 900
o
C has not yet occurred completely.The xrd
result of sample calcined 1000
o
C indicate single
phase of SrFe
12
O
19
with hexagonal crystal structure,
in this case the hematite (Fe
2
O
3
) does not appear.
The measurement of magnetic properties was done
using Vibrating Sample Magnetometer (VSM) in
evaluation of magnetic behaviour between xrd result
with remanence and coercivity. The hysteresis loop
from VSM is shown at Figure 4 for sample after
calcination at 900 and 1000
o
C.
Figure 3: The XRD patterns of sample after calcination at
900
o
C and 1000
o
C.
The phase identification was done using Match
soft ware through matching experiment data with
reference data. The xrd result show that it is found
two peaks namely hematite (Fe
2
O
3
) phase and
SrFe
12
O
19
phase at sample after calcination at 900
o
C.
It is indicated that the starting of forming phase is at
temperature 900
o
C, but the reaction of SrF formation
at 900
o
C has not yet occurred completely.Thexrd
result of sample calcined 1000
o
C indicates a single
phase of SrFe
12
O
19
with hexagonal crystal structure,
in this case the hematite (Fe
2
O
3
) does not appear.
The measurement of magnetic properties was done
using Vibrating Sample Magnetometer (VSM) to
evaluate magnetic behavior between xrd result with
remanence and coercivity. The hysteresis loop from
VSM is shown at Figure 4 for sample after
calcination at 900 and 1000
o
C. Hysteresis loop
curves as seen in Figure 4 for both samples show a
hysteresis loop for permanent magnets material,
which permanent magnet material has a wide
hysteresis loop or has a coercivity value greater than
100 Oe.
Figure 4: Hysteresis loop of samples after calcination at
900 and 1000
o
C.
Sample calcined at 900
o
C has magnetic
properties (remanence, coercivity and magnetic
saturation) lower than sample calcined at 1000
o
C, it
is due to existing of hematite (Fe
2
O
3
) phase, which
Fe
2
O
3
is classified as soft magnet materials and
sample calcined 1000
o
C has only single phase of
SrFe
12
O
19
, The value of remanence, coercivity and
magnetic saturation for both samples are shown at
Table 1.
Table 1: Value of remanence (mr), coercivity (Hcj) and
Magnetic saturation (ms).
Sam
p
le mr, emu/
g
Hc
j
, Oe Ms, emu/
g
Calcination
900
o
C
180 1900 500
Calcination
1000
o
C
390 3000 715
The highest value of magnetic properties is achieved
at sample calcined at 1000
o
C i.e. the value of mr =
390 emu/g, Hcj about 3000 Oeand ms = 715 emu/g,
because this sample has a single phase SrFe
12
O
19
. If
the coercivity value from this experiment (3000 Oe
or 239 kA/mm) compared to the theoriticalcoercivity
for Sr ferrite is slightly lower (Slusarek &
Zakrzewski, 2012).
4 CONCLUSSION
Magnetic powder of SrFe
12
O
19
with hexagonal
crystal structure can be made by powder metallurgy
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
462

technique which the single phase of SrFe
12
O
19
is
achieved at calcination temperature about 1000
o
C.
Magnetic powder SrFe
12
O
19
is as a permanent
magnet materials with remanence value about 390
emu/g, coercivity value about 3000 Oe and magnetic
saturation about 715 emu/g.
ACKNOWLEDGEMENTS
Authors wishing to acknowledge a work by
technical staff XRD, PSA and VSM from Research
Center for Physics LIPI and thank you for funding
support by University Sriwijaya especially LPPM
and Ristekdikti at “Program Riset Kompetitip”.
REFERENCES
Ghobeiti-Hasab, M. (2014). Production of Sr-Ferrite Sub-
Micron Powder by Conventional and Sol-Gel Auto-
Combustion Methods. International Journal of
Chemical, Nuclear, Metallurgical and Materials
Engineering, 8(12), 1245–1248.
Mahmood, S. H., & Abu-Aljarayesh, I. (2016).
Hexaferrite Permanent Magnetic Materials. Materials
Research Forum LLC.
https://doi.org/http://dx.doi.org/10.21741/9781945291
074
Moosa, I. S. (2013). Powder Metallurgy and its
Application in The Production of Permanent Magnets.
International Journal of Advanced Research in
Engineering and Technology, 4(6), 128–138.
Nowosielski, R., Babilas, R., Dercz, G., & Pająk, L.
(2008). Microstructure of polymer composite with
barium ferrite powder. Journal of Achievements in
Materials and Manufacturing Engineering, 31(2),
269–274.
Nowosielski, R., Babilas, R., Dercz, G., Pająk, L., &
Wrona, J. (2007). Structure and properties of barium
ferrite powders prepared by milling and annealing.
Archives of Materials Science and Engineering,
28(12), 735–742.
Pullar, R. C. (2012). Hexagonal ferrites: A review of the
synthesis, properties and applications of hexaferrite
ceramics. Progress in Materials Science, 57(7), 1191–
1334.
Sebayang, P., Masbah, M., Siregar, R. T., & Walyo, T. B.
(2011). Ferrite-based material as a permanent magnet
for components of electrical generators. Advances in
Natural Sciences: Nanoscience and Nanotechnology,
2(4), 1–5.
Slusarek, B., Karbowiak, M., Jankowski, B., Kapelski, D.,
& Przybylski, M. (2013). Physical properties of
permanent magnets for magnetic circuits of electric
machines. Polimery, 58(2), 127–134.
https://doi.org/http://dx.doi.org/10.14314/polimery.20
13.127
Slusarek, B., & Zakrzewski, K. (2012). Magnetic
properties of permanent magnets for magnetic sensors
working in wide range of temperature. Przeglad
Elektrotechniczny (Electrical Review), 88(7), 123–
126.
Takahashi, M., Sugiura, N., Ushigami, Y., Hara, T.,
Uemori, R., Shirahata, H., Mizoguchi, M., Uenishi, A.,
& Kojima, K. (2012). Metallurgical Approaches for
Product Development and Process Optimization. In
Nippon Steel Technical Report (Issue 101).
Synthesis and Characterization of Strontium Hexa Ferrite (SrFe12O19) Powder by using Powder Metallurgy Method
463
