
The Influence of the Ethanol Extract of Bitter Vine (Mikania
micrantha Kunth.) on the Mortality, the Hatchability of the Eggs and
the Larval Growth of Aedes aegypti Linn.
Nursal
1*
and A. Hardiansyah
1
1
Department of Biology, Universitas Sumatera Utara, Jl. Dr. T. Mansyur No.9, Medan, Indonesia
Keywords: Mikania micrantha, Mortality, Growth, Aedes Aegypti.
Abstract: The research on the impact of the ethanol extract of M. micrantha leaf on the mortality, egg hatchability and
larval growth of A. aegypti had been conducted using a Complete Randomized Design (CDR) with five
treatments and replications. The mortality tests on 3rd instar larva with concentration treatments of the ethanol
extract of M. micrantha leaves at 0.2%,0.4%,0.6%,0.8%, and 0.1%generated a LC
50
value of 0.58%.The
ethanol extract of M. micrantha leaves at a sub-lethal concentration of 0,1%,0,2%,0,3%,0,4%,0,5%indicated a
significant impact on the mortality, egg hatchability and larval growth (p≤0,05).A sublethal concentration at
0,4% of the plant was effective in suppressing the egg hatchability at a percentage of 41,6%, larval
development into pupa at a percentage of 19.5% and pupae transformation into imago a percentage of
63,3%.
1 INTRODUCTION
The control of the vector-borne disease can be
conducted using chemical compounds such as
synthetic insecticides, but this may cause losses such
as resistance, death of untargeted, or human
poisoning. World Health Organization has
advocated finding alternatives to control these issues
through biological or environmental control
methods, by using natural chemicals derived from
the plants (Indonesian Department of Health, 2010).
Floras in Indonesia have mass potentials to be
utilized as an alternative for plant-based insecticides
using the secondary metabolites they produce
(Boesri et al., 2015). Organic insecticides are
generally pesticides whose active ingredients come
from plant parts that are toxic to insects and have
secondary metabolites containing various bioactive
compounds (Thamrin, M. et al., 2007).
Various plants, including weeds, have secondary
metabolite compounds that can be used for self-
defense against pests and diseases (Tampubolon,
2018). Bitter Vine (Mikania micrantha) is one of the
potential weeds and has been proven as an effective
plant-based insecticide because it contains secondary
metabolites that can kill insects (Salam et al., 2014).
Based on the phytochemical analysis results, the
leaf extract of M. micrantha contains active
substances in the form of secondary metabolites
such as alkaloids, saponins, flavonoids, steroids,
tannins, and terpenoids (Polakitan et al., 2017). M.
micrantha also has other specific active substances
called mikanolide and dihydromichiolide. These
substances belong to the sesquiterpene group
commonly found in the plants of the Asteraceae
family (Tripathi et al., 2012). Noshirma & Willa
(2016), in their research, mentioned that these
phenolic metabolites might cause stomach poisoning
which can interfere the digestive system of A.
aegypti larvae, so the larvae fail to develop and
eventually die. “interfere with the digestive system”
Plant-based insecticides also work specifically
by damaging the growth of eggs, larvae, and pupae;
inhibiting skin turnover; disrupting insect
communication; inhibiting the female reproduction;
reducing appetite; blocking the insect ability to eat;
and repelling the insects (Sudarmo, 2005). Only a
few studies on the leaves of M. micrantha as an
organic insecticide have been conducted, so a test is
required to see the effect on the egg hatchability,
mortality and development of A. aegypti larvae as
one of the efforts in controlling the number of A.
aegypti mosquitoes through monitoring in the larval
450
Nursal, . and Hardiansyah, A.
The Influence of the Ethanol Extract of Bitter Vine (Mikania micrantha Kunth.) on the Mortality, the Hatchability of the Eggs and the Larval Growth of Aedes aegypti Linn..
DOI: 10.5220/0010204500002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 450-455
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

phase. Thus, the object of this study was how the
ethanol extract of M. micrantha leaves affect the egg
hatchability, mortality, and development of A.
aegypti larvae. The study was conducted to
determine the effect of ethanol extract of M.
micrantha leaves on larval mortality, egg
hatchability, and development of A. aegypti larvae.
2 METHODS
2.1 Animal Subject Rearing
Rearing was carried out to keep and breed the
animal subjects to provide the eggs and larvae of the
A. aegypti. It was conducted at the Institute of
Environmental Health and Infection Disease Control
(BTKL-PPM) Class 1 Medan.
2.2 General Architecture
This research is an experiment using a completely
randomized design (CRD) with five extract
concentrations (with one control) and five iterations
of 25 larvae and A. aegypti eggs.
2.3 Ethanol Extract of M. micrantha
Leaves
Five kilograms of M. micrantha leaf samples were
washed and dried for five days, then crushed using a
blender to form a powder. The powder was weighed
as much as 1 kg then macerated with ethanol for 144
hours and stored in Erlenmeyer. During the
maceration process, the stirring was carried out
every day until obtained macerate. The obtained
macerate was then filtered and evaporated with a
Vacuum Rotary Evaporator until all the ethanol
evaporated into a thick extract. This extract would
be stored in a silica gel desiccator (Hamidah et al.,
2015).
2.4 Observation of Test Parameter
2.4.1 The Mortality Test on the Third Instar
A. aegypti Larva
The toxicity test of the ethanol extract of M.
micrantha leaves on the mortality of the third instar
A. aegypti larvae was conducted using six
concentration variants with five replications, namely
K1 = 0.2%, K2 = 0.4%, K3 = 0.6%, K4 = 0,8%, K5
= 1%, and K0 = 0% (control). A total of 25 third
instar A. aegypti larvae were put into separate
exposure medium containing 100 ml of each extract.
The temperature and humidity in the treatment room
as and the exposure medium were set as the standard
measurement. The observation was performed 24
hours after the exposure. The larval mortality rate
can be calculated using the formula below.
𝐿𝑎𝑟𝑣𝑎𝑙 𝑀𝑜𝑟𝑡𝑎𝑙𝑖𝑡𝑦
%
𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝐷𝑒𝑎𝑑 𝐿𝑎𝑟𝑣𝑎𝑒
𝑇𝑜𝑡𝑎𝑙 𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝐿𝑎𝑟𝑣𝑎𝑙 𝑆𝑢𝑏𝑗𝑒𝑐𝑡𝑠
𝑥 100
(1)
Furthermore, the da
ta analyzed for regression to
obtain the LC50 value using Microsoft Excel 2013.
2.4.2 Egg Hatchability and Growth of A.
aegypti Larvae at Sublethal
Concentration
The sublethal test was conducted to determine the
egg hatchability and larval development of A.
aegypti until the imago phase. Twenty-five eggs of
A. aegypti were put into a test cup containing 100 ml
of ethanol extract of M.micrantha leaf with sublethal
concentrations, based on the results of the mortality
test, at P0 = 0% (control), P1 = 0.1%, P = 2, 0.2%,
P3 = 0.3%, P4 = 0.4% P5 = 0.5% with five
replications. The observation of the egg hatchability
was monitored every 24 hours for 72 hours (3 days)
(WHO, 2005).
𝐸𝑔𝑔 𝐻𝑎𝑡𝑐ℎ𝑎𝑏𝑖𝑙𝑖𝑡𝑦 %
𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 ℎ𝑎𝑡𝑐ℎ𝑒𝑑 𝑒𝑔𝑔𝑠
𝑇𝑜𝑡𝑎𝑙 𝑜
𝑓
𝑒
𝑔𝑔
𝑠
𝑥 100
(2)
The testing of the development of A. aegypti was
observed from the hatched eggs into larval stages in
24 hours for ten days. It aims to determine the
number of successful larvae
transformed into pupae
and pupae into the imago. This can be calculated
using the formulas below.
𝐿𝑎𝑟𝑣𝑒 𝑃𝑢𝑝𝑎𝑒
%
𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝐷𝑒𝑣𝑒𝑙𝑜𝑝𝑒𝑑 𝑃𝑢𝑝𝑎𝑒
𝑇𝑜𝑡𝑎𝑙 𝑜
𝑓
𝐿𝑎𝑟𝑣𝑎𝑒
𝑥 100
(3)
𝑃𝑢𝑝𝑎𝑒 𝐼𝑚𝑎𝑔𝑜
%
𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝐷𝑒𝑣𝑒𝑙𝑜𝑝𝑒𝑑 𝐼𝑚𝑎𝑔𝑜
𝑇𝑜𝑡𝑎𝑙 𝑜
𝑓
𝑃𝑢𝑝𝑎𝑒
𝑥 100
(4)
2.5 Statistical Analysis
The obtained data from each observation variables
were recorded and arranged in tabular form. The
The Influence of the Ethanol Extract of Bitter Vine (Mikania micrantha Kunth.) on the Mortality, the Hatchability of the Eggs and the Larval
Growth of Aedes aegypti Linn.
451
generated quantitative data (dependent variables)
were tested for their significance on the impact of
the treatment groups (independent variables) with
the help of a statistical computer program, namely
the SPSS (release 22). The test sequence began with
a normality test, homogeneity test, one-way
ANOVA test for data with repeated observations
(more than two times).
If in the ANOVA test, there is a significant
difference (p <0.05) in the treatment group, then the
test will be continued using the Post Hoc-Duncan
analysis at a level of 5%. At the end of the study, it
can be determined which concentration of ethanol
extract of M. micrantha leaves has the most
significant and practical effect on the egg
hatchability and larval growth of A. aegypti.
3 RESULTS AND DISCUSSION
3.1 Larval Mortality
Based on the test results, the mortality rate of the
third instar A aegypti larvae was obtained after
being treated with ethanol extract of M. micrantha
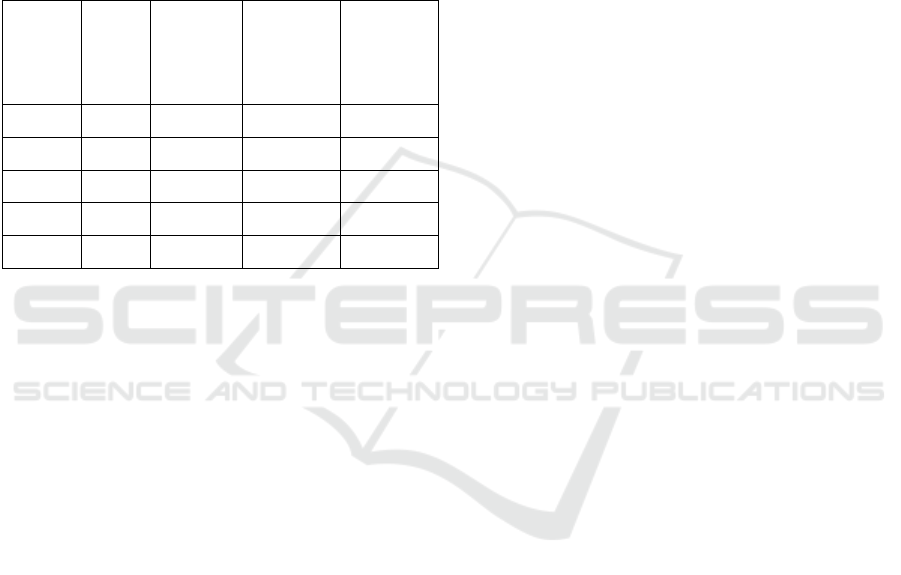
leaves. It can be seen in Figure 1.
Figure 1: Influence of ethanol extract of M. micrantha
leaves on the mortality rate of the third instar A. aegypti
larvae (24-hour observation). Note: K0: 0% (control), K1:
0.2%, K2: 0.4%, K3: 0.6%, K4: 0.8% and K5: 1%. The
number followed by the same letter in the figure was not
significantly different in the Duncan test at a rate of 5%.
Figure 1 shows that the greater the concentration
of the treatment given, the higher the percentage of
larval mortality rate, compared to the K0 (control)
treatment. In the K0 treatment, no larvae mortality
was found. Larval mortality began to occur in the
K1 treatment with the lowest percentage of mortality
rate at 16%, while the highest mortality was in the
K5 treatment at a percentage of 88%. Based on the
results shown in Figure 1, the lethal concentration
value of 50% (LC50) can be determined by using
probit analysis.
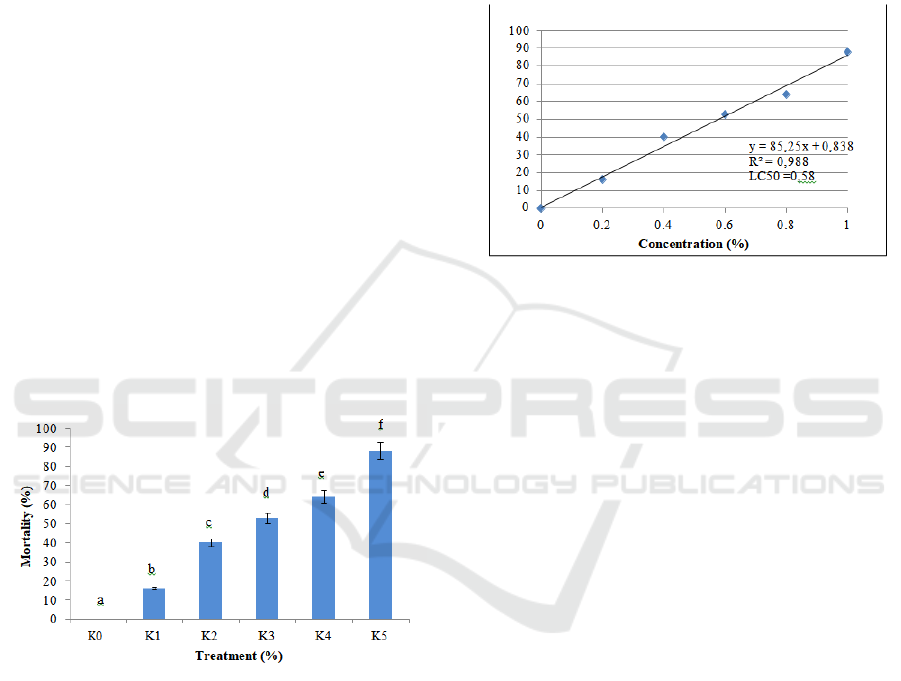
The result of the probit analysis showed that the
LC50 value is at 0.58% after 24 hours. The
regression calculations illustrated the relationship
between the extract concentration of M. micrantha
leaf and larval mortality. This can be obtained using
the equation of y = 0.44 + 21.0x with a regression
coefficient of r2 = 0.98, which is shown in Figure 2.
Figure 2: Graph of regression analysis on the effect of the
ethanol extract concentration of M. micrantha leaves with
the percentage of A. aegypti larval mortality.
Fitmaya (2006) stated that the higher the
concentration of the insecticide given, the higher the
content of the active substance so it can increase
metabolic obstruction of the larvae subject which led
to the increasing percentage of its death. Haisya, N.
et al., (2013) mentioned that the leaves of M.
micrantha contain several active compounds in the
form of secondary metabolites such as alkaloids,
flavonoids, tannins that are insecticides.
Each active substance has different work
principles in impacting larval mortality. According
to Cania (2013), alkaloids serve as a stomach
poison. Alkaloids, in the form of salts, can degrade
the cell membranes to damage the cells and also
disrupt the larval nerve system by inhibiting the
action of the acetylcholinesterase enzyme and
causing larvae to undergo paralysis and die.
Flavonoids act as a respiratory poison, causing
larvae death. Tannins play a role in reducing the
ability to digest the food by suppressing the activity
of digestive enzymes (Haditomo, 2010).
The lower the LC50 value of a substance means
that the substance has higher activity in killing
experimental animals since it requires a lower
concentration to kill the animals simultaneously
(Chang, 2004).
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
452
3.2 Egg Hatchability and Development
of A. aegypti Larvae in Sublethal
Concentration
The tests on egg hatchability and larval development
were performed using LC
50
at sublethal
concentrations (0.58%) of 0.5%, 0.4%, 0.3%, 0.2%
and 0.1%.
Table 1: The influence of ethanol extract of M. micrantha
leaf on the percentage of the egg hatchability, and the
larvae-pupae and pupae-imago growth of A. aegypti in
the 14-days observation.
Treatm
ent
Numb
er
Egg
Hatchabil
ity (%)
Larvae-
Pupae
Developm
ent
(%)
Pupae –
imago
Developm
ent
(%)
P0 25
92
a
85.3
a
92.0
a
P1 25
80.8
b
68.4
b
87.0
b
P2 25
73.6
b
66.4
b
82.3
b
P3 25
65.6
c
44.3
c
78.3
c
P4 25
41.6
d
19.5
d
63.3
d
Note that P0= 0 % (control), P1= 0.1%, P2=
0.2%, P3= 0.3% , P4= 0.4%, and P5= 0.5%. The
number followed by the same letter in the
same
column
was not significantly different in the
Duncan test at a rate of 5%.
3.2.1 The Egg Hatchability
Table 1. shows that the ethanol extract of M.
micrantha leaf at treatments of P1, P2, P3, P4, and
P5 can decrease the rate of the egg hatchability, and
development of larvae-pupae and pupae-imago
compared to P0 (control). The number of hatched
eggs decreases with increasing concentration of the
given treatment. The lowest number of hatched eggs
was at P5 treatment at a percentage of 26.4%, while
the highest hatchability rate at P1 treatment at
80.8%.
The difference in egg hatchability is thought to
be caused by the secondary metabolite content of the
extract which disrupts the metabolism in the egg, so
the egg fails to hatch. Salam et al., (2014) stated that
M. micrantha leaves contain active substances such
as flavonoids and tannins. The decline in the
percentage of the egg hatchability is due to the
flavonoids that enter the egg through the diffusion
process on the surface of the eggshell.
3.2.2 Development of Larvae - Pupae
Table 1 showed that the ethanol extract of M.
micrantha leaf in all treatments was able to suppress
the development of the larvae-pupa stage. The
highest percentage of the successful growth of
larvae-pupa was in P1 treatment at 68.4% while the
lowest occurred in P5 treatment at 11.5%. This
shows that during exposure, the ethanol extract of
M. micrantha leaves affected the development of
larvae into pupae. The higher concentration of
ethanol extract of M. micrantha leaves led to the
failure of larvae to become pupae, thus reducing the
percentage of successful development
During the observation, the larval phase showed
a tendency to require a longer time to develop into
pupae, which was around 10-12 days, compared to
the control treatment, which only took 8-10 days.
The larvae also tend to experience changes in body
size which is larger than the larvae in the control
treatment group. This may be caused by the
extracted content of M. micrantha leaf, in the form
of Mikanolide, and belongs to the sesquiterpene
group, which has a structural similarity to the
juvenile hormone.
Bowers (1971) stated that the metamorphosis
stage from larvae to pupae is sensitive and complex.
The distribution of juvenile hormones in a long time
can have effects such as the formation of large
larvae. Elimam et al., (2009) also revealed that the
levels of the juvenile hormone could directly
determine the larval stage to become a pupa that will
last a long time.
3.2.3 Development of Pupae - Imago
The living pupae were observed their development
into the imago. The percentage of pupa-imago was
obtained from the comparison of the number of
imagoes formed with the total number of pupae.
Table 1 shows that the highest percentage of
successful pupa-imago development was in the P1
treatment at 87.0%, while the lowest was in the P5
treatment at 50.0%. All ethanol extracts of M.
micrantha leaves tended not to affect the percentage
of developmental failure of the pupae-imago stage
compared to the control treatments.
This can be seen from the high percentage of
pupae-imago. This may be related to the pupae that
no longer needs food, which means that the pupae
do not consume the extract solution anymore so that
the pupa can avoid the toxic effects of the ethanol
extract of M. micrantha leaves.
During the observation, it could be seen that the
growth of pupae to imago needed a longer time,
The Influence of the Ethanol Extract of Bitter Vine (Mikania micrantha Kunth.) on the Mortality, the Hatchability of the Eggs and the Larval
Growth of Aedes aegypti Linn.
453

which was around 5-6 days. (Yulidar & Wilya,
2015) stated that the normal time for pupae to
develop into imago is 3-4 days.
The pupae stage is a fasting phase where the
body is wrapped in a layer called the puparium. The
increase in the time it takes for a pupa to become an
imago may be due to the pupa trying to survive by
extending its maturation period into an imago, so the
pupa remains protected by a protective layer that
wraps its body from exposure to toxic extracts.
Typically, at the fourth instar larvae, there is a
decrease in the secretion of juvenile hormone by
corpora allata, but with the exposure to the ethanol
extract of M. micrantha leaves, which was thought
to have an effect like juvenile hormone, induced the
pupae to prolong its development into an imago
(Habibi, 2011).
Based on the results of the study on the effect of
the ethanol extract of M. micrantha leaves on the
egg hatchability and the development of A. aegypti,
it can be determined that the most significant
concentration which can reduce the rate of the egg
hatchability and the larval development is the P4
treatment with a concentration of 0.4 %.
The same thing occurred in the research of
Nursal & Hardiansyah (2018). The dichloromethane
extract of the leaves of the bitter melon (Momordica
charantia L.), basil (Ocimum basilicum L.), and
lemongrass (Cymbopogon winterianus) can reduce
the percentage of the egg hatchability and the larval
development (larvae- pupae and pupae – adult) of
Aedes aegypti. Likewise, with Nursal & Yeanny
(2019), the ethanol extract of the leaves of bitter
melon (Momordica charantia L.) and basil (Ocimum
basilicum L.) can also reduce the hatchability of the
eggs and the growth (larvae-pupae and pupae-adult)
of Aedes aegypti mosquitoes.
4 CONCLUSIONS
Based on the test results, it can be concluded that the
LC50 concentration of the ethanol extract of M.
micrantha leaves on the mortality of third instar
larvae was at 0.58%. The sublethal concentration of
the ethanol extract of M. micrantha leaves has a
significant influence on the eggs hatchability and
larval development. The ethanol extract
concentration of M. micrantha at 0.4% was effective
in reducing the rate of the eggs hatchability and the
larval development into pupae, and the pupae
development into imago by 41.6%, 19.5%, and
63.3% respectively.
REFERENCES
Boesri, H., Heriyanto, B., Wahyuni, S., Handayani, &
Suwaryono, T. (2015). Toxicity Test of Numerous
Plant Extracts against Aedes aegypti Larvae Dengue
Hemorrhagic Fever Vector. Vektora, 7, 29–38.
Bowers, W. S. (1971). Insect Hormones and Their
Derivatives as Insecticides. Bull. Org. Mond. Sante;
Bull. Wld Hlth Org, 44, 381–389.
Cania, E. (2013). The Effectiveness of Larvacides Legundi
Leaf Extract (Vitex trifolia) Against Aedes aegypti
Larvae. Medical of Journal Lampung University,
22(4), 52–60.
Chang, P. S. (2004). Cinnamon Oil May Be an
Environmentally Friendly Practice, With the Ability to
Kill Mosquito Larvae.
Elimam, A. M., Elmalik, K. H., & Ali, F. (2009).
Larvicidal Adult Emergence Inhibition and
Oviposition Deterrent Effects of Foliage Extract from
Ricinus communis L. against Anopheles arabiensis
and Culex quinquefasciatus in Sudan. Tropical
Biomedicine, 26(2), 130–139.
Fitmaya, A. (2006). The Activity Test of the Ethanol
Extract Larvalide at 96% of Sweet Starfruit (Averrhoa
carambola L.) Leaves against the Third Instar
Anopheles aconitus Larvae and its Thin Layer
Chromatography. Universitas Muhammadiyah
Surakarta.
Habibi, S. (2011). Juvenile Hormone (JH) As a Supporter
And Controller Of Insect Life. Proceedings of
National Seminar of Universitas Terbuka Banten.
Haditomo, I. (2010). The Effect of Larvaside Extract of
Clove (Syzygium aromaticum L.) Leaves Against
Aedes aegypti L. Universitas Sebelas Maret.
Haisya, N., Asfi, R. L., & Riris, P. S. (2013). Bitter Vine
(Mikania micrantha H.B.K.) as natural alternative
antibacterial and its study against bacterial common
as a causative agent in cattle mastitis in Indonesia.
Hamidah, H. S., Mukarlina, L., & Linda, R. (2015). The
ability of the extract of the convex vine (Mikania
micrantha H.B.K) leaves as a bioherbicide of Weed
Melastoma affine D.Don. Protobiont, 4(1), 89–93.
Indonesian Department of Health. (2010). Epidemiology
Window Bulletin Topics of Dengue Hemorrhagic
Fever (2nd ed.). Surveillance and Epidemiology Data
Center.
Noshirma, M., & Willa, R. W. (2016). Biological
larvicides used in the Control of Dengue Fever Vector
in Indonesia. SEL, 3(1), 31–40.
Nursal, & Hardiansyah, A. (2018). The effectiveness of
dichloromethane extract of various plants on eggs
hatchability, and life cycle of Aedes aegypti L.
mosquitoes. Journal of Physics: Conference Series.
Nursal, & Yeanny, S. M. (2019). The Egg Hatchability
and the Development of Aedes aegypti Mosquitoes in
Ethanol Extracts of the Leaves of Bitter Melon
(Momordica charantia L.) and Basil (Ocimum
basilicum L.). IOP Conference Series: Earth and
Environmental Science.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
454

Polakitan, I. R., Fatimawali, & Leman, M. A. (2017).
Inhibitory Test of the Extract of Bitter Vine (Mikania
micrantha) against Streptococcus mutans Growth.
Pharmacon, 6(1), 1–8.
Salam, D. M., Mukarlina, L., & Diba, F. (2014).
Coptotermes curvignathus Holmgren Soil Termite
Control Using The Extracts of Bitter Vine Leaf
(Mikania micrantha Kunth.). Protobiont, 3(2), 87–92.
Sudarmo, S. (2005). Plant-Based Pesticides. Kanisius
Publishing.
Tampubolon, K. (2018). The Potential of Secondary
Metabolites of Weeds as Organic Pesticides in
Indonesia. Journal of Cultivation, 17(3), 683–693.
Thamrin, M., Asikin, S., Mukhlis, & Budiman, A. (2007).
Potential of Swamp Land Flora Extract as Plant-Based
Pesticides. Swamp Field Research Center, 35–54.
Tripathi, R. S., Khan, M. L., & Yadav, A. S. (2012).
Biology of Mikania micrantha H.B.K. A Review.
WHO. (2005). Guidelines for laboratory and field testing
of mosquito larvicide.
Yulidar, & Wilya, W. (2015). Life Cycle of Aedes
Aegypti on Laboratory Scale. SEL, 2(1), 22–28.
The Influence of the Ethanol Extract of Bitter Vine (Mikania micrantha Kunth.) on the Mortality, the Hatchability of the Eggs and the Larval
Growth of Aedes aegypti Linn.
455
