
Isolation and Determination of Amylase Enzyme Activity from
Durian Seed Sprouts
M. Zulham Efendi Sinaga
1,2
, Cut Fatimah Zuhra
1,2
, Emma Zaidar
1,2
, Firman Sebayang
1
,
Rumondang Bulan
1
and Dea Rahmadana
1
1
Department of Chemistry, Universitas Sumatera Utara, Medan, Indonesia
2
Pusat Unggulan Iptek (PUI) Kitosan dan Material Maju
Keywords: Enzyme, Amylase, Germination, Durian Seed Sprouts.
Abstract: One of the favorite fruits in Medan-Indonesia is durian. The number of fruit is comparable to the most waste
of durian seed produced. Seeds are one of the ways to breed through the process of germination. In the
process of germination which plays an important role is the enzyme amylase. Based on this, the research
aims to isolate the enzyme amylase found in durian seed sprouts on the fifth day through dialysis with
ammonium sulfate and then determined its activity against temperature, pH, substrate concentration.
Through this research, the enzyme amylase has been isolated from the sprouts of durian beans with the best
activity at temperature 40 oC (36.87 U/ml), pH 7 (36.86 U/ml), substrate concentration 1% (26.36 U/ml).
The best ammonium sulfate saturation in the dialysis process is 60% acquired enzyme activity of 58.59
U/ml.
1 INTRODUCTION
Enzyme is a biocatalyst which can accelerate the
process of a reaction. Currently, the use of enzymes
in an industrial field is increasing. Some industries
that use enzymes among others are pharmaceutical
industry, food and beverage industry, and energy
sector (Chapman et al., 2018). One of the most
widely used enzymes is the amylase enzyme.
Amylase enzyme is an enzyme that hydrolyzes
starch to dextrin, maltose and glucose units by
cutting glycosidic bonds α- 1,4 and α- 1,6 in starch
(Mohanan & Satyanarayana, 2018; Simair et al.,
2017). In the food industry, amylase enzymes play
an important role in producing syrups and
sweeteners, in baking, in cereals and also in
beverage production. The wide use of amylase
enzymes in industry makes this amylase enzyme
take up 25% of the world's market enzymes (Naili et
al., 2016; Sindhu et al., 2017). The large enzyme
needs make researchers have to find new sources to
produce the enzyme amylase.
One of the process which requires amylase
enzymes is the germination process, in the
germination process the amylase enzyme is needed
to produce energy that will be used for the growth
process (Joshi, 2018). Based on these reasons, the
potential for durian seed sprouts as a source of
amylase enzymes is considered to have great
potential. In this study the age of durian seed sprouts
used as a source of the amylase enzyme was 5 days
old. Some factors that determine enzyme activity are
enzyme purity, pH, temperature and substrate
concentration of the enzyme. Therefore in this study
the parameters to be tested are the activity of the
amylase enzyme isolated from durian seed sprouts
on variation of temperature, pH and substrate
concentration. After the optimum conditions are
obtained then it will be applied to determine the
enzyme activity after the purification process by
using several variations of ammonium sulfate.
The success of this research is expected to add
new information on the source of the amylase
enzyme from durian seed sprouts. The potential of
durian is very much in Medan will add another
advantage of the waste produced by the durian fruits.
Sinaga, M., Zuhra, C., Zaidar, E., Sebayang, F., Bulan, R. and Rahmadana, D.
Isolation and Determination of Amylase Enzyme Activity from Durian Seed Sprouts.
DOI: 10.5220/0010199800002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 413-417
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
413

2 MATERIALS AND METHODS
2.1 Isolation of Amylase Enzyme from
Durian Seed Sprouts
Durian seeds are washed with running water and
then put into a container for the germination process,
prepared by growing media and then waited for
durian seed germination for 5 days. 150 grams of 5
days durian seed sprouts obtained were
homogenized with 250 ml of 1% Isotonic NaCl
solution in a cold state. Then mashed and filtered
until the filtrate and pulp separate. The filtrate was
centrifuged at 10,000 rpm at 20C for 10 minutes.
The crude of enzyme produced was tested for its
activity against temperature variations (30, 35, 40,
45 dan 50C), pH (3, 5, 7, dan 9) and Substrate
concentration (0,5; 1; 1,5; 2; dan 2,5% w/v). The
resulting enzyme crude is purified by varying the
saturation level of ammonium sulfate (20, 40, 60 dan
80% w/v) then the purification results of each
variation were tested their activity back to the
optimum temperature, pH and substrate
concentrations to determine the activity of the
resulting amylase enzyme after purification.
2.2 Crude Amylase Enzyme Activity
Test on Variation of Temperature
0.5 mL of 1% starch solution was put into the test
tube then added 5 mL of buffer phosphate pH 7.
Added 1 mL of crude amylase enzyme extract and 1
ml of 1% NaCl. Then incubated at temperature
variations (30, 35, 40, 45 and 50 0C) for 1 hour.
After that, 1 mL of 0.1N NaOH was added and
centrifuged at 3400 rpm for 20 minutes. 1 ml of the
supernatant is taken and then diluted in 10 ml
measuring flask and then homogenized. Put 1 ml of
dilution results into the test tube then add 1 ml of
Nelson’s reagent and heated in a water bath for 20
minutes. Then removed and cooled until the
temperature 25 0C. 0.5 mL of arsenomolybdate was
added and then shaken until all the sediment
dissolved. Then added 7 mL of distilled water and
then shaken until homogeneous. Its absorption is
measured at a wavelength of 645 nm.
2.3 Crude Amylase Enzyme Activity
Test on Variation of pH
0.5 mL of 1% starch solution was put into the test
tube then added 5 mL of buffer phosphate pH 3, 5, 7
and 9. Added 1 mL of crude amylase enzyme extract
and 1 ml of 1% NaCl. Then incubated at optimum
temperature for 1 hour. After that, 1 mL of 0.1N
NaOH was added and centrifuged at 3400 rpm for
20 minutes. 1 ml of the supernatant is taken and then
diluted in 10 ml measuring flask and then
homogenized. Put 1 ml of dilution results into the
test tube then add 1 ml of Nelson’s reagent and
heated in a water bath for 20 minutes. Then removed
and cooled until the temperature 25C. 0.5 mL of
arsenomolybdate was added and then shaken until
all the sediment dissolved. Then added 7 mL of
distilled water and then shaken until homogeneous.
Its absorption is measured at a wavelength of 645
nm.
2.4 Crude Amylase Enzyme Activity
Test on Variation of Substrate
Concentrations
0.5 mL of starch solution variation (0,5; 1; 1,5; 2;
dan 2,5%) was put into the test tube then added 5
mL of buffer phosphate optimum pH. Added 1 mL
of crude amylase enzyme extract and 1 ml of 1%
NaCl. Then incubated at optimum temperature for 1
hour. After that, 1 mL of 0.1N NaOH was added and
centrifuged at 3400 rpm for 20 minutes. 1 ml of the
supernatant is taken and then diluted in 10 ml
measuring flask and then homogenized. Put 1 ml of
dilution results into the test tube then add 1 ml of
Nelson’s reagent and heated in a water bath for 20
minutes. Then removed and cooled until the
temperature 25C. 0.5 mL of arsenomolybdate was
added and then shaken until all the sediment
dissolved. Then added 7 mL of distilled water and
then shaken until homogeneous. Its absorption is
measured at a wavelength of 645 nm.
2.5 Crude Amylase Enzyme Activity
Test after Purification with
Ammonium Sulfate
0.5 mL of starch solution optimum variation was put
into the test tube then added 5 mL of buffer
phosphate optimum pH. Added 1 mL of crude
amylase enzyme extract after purification with
variations in the saturation level of ammonium
sulfate (20, 40, 60 dan 80%) and 1 ml of 1% NaCl.
Then incubated at optimum temperature for 1 hour.
After that, 1 mL of 0.1N NaOH was added and
centrifuged at 3400 rpm for 20 minutes. 1 ml of the
supernatant is taken and then diluted in 10 ml
measuring flask and then homogenized. Put 1 ml of
dilution results into the test tube then add 1 ml of
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
414
Nelson’s reagent and heated in a water bath for 20
minutes. Then removed and cooled until the
temperature 25C. 0.5 mL of arsenomolybdate was
added and then shaken until all the sediment
dissolved. Then added 7 mL of distilled water and
then shaken until homogeneous. Its absorption is
measured at a wavelength of 645 nm.
3 RESULTS AND DISCUSSIONS
Durian seeds used in this study were obtained from
durian traders around Medan, North Sumatera,
Indonesia. Durian seeds that have been cleaned are
then germinated, after 5 days the resulting sprouts
will then be processed as a source of the amylase
enzyme. Germination carried out in this study after 5
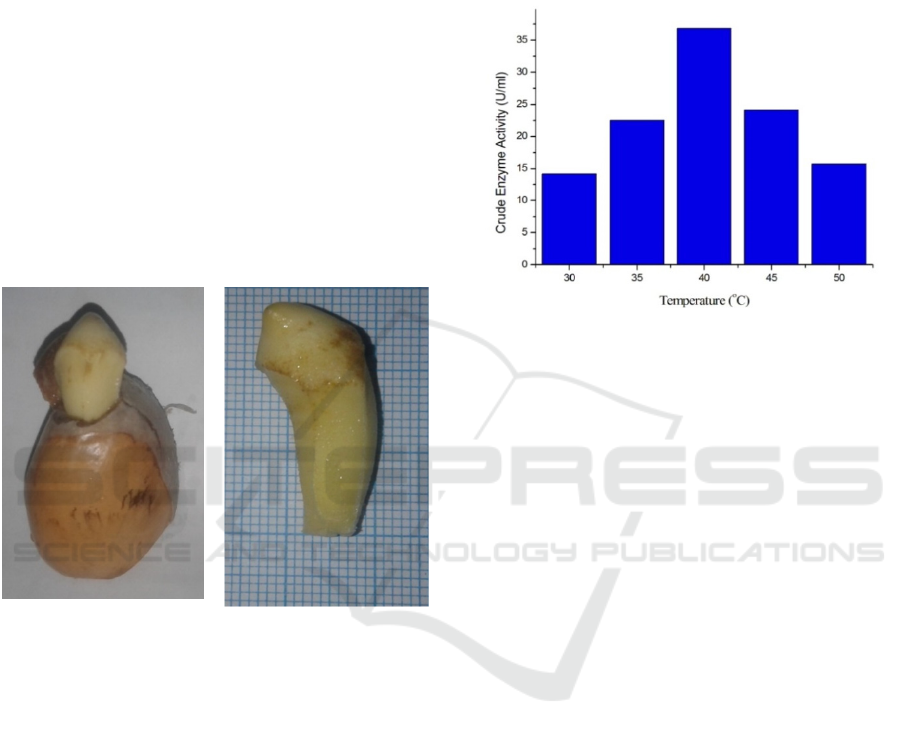
days as in Figure 1 below.
Figure 1: Durian Seed Sprouts.
Based on Figure 3.1 above durian seed sprouts
after 5 days have an average height of 2.5 cm - 2.8
cm. Some factors that can affect the process of
germination development are temperature, water
potential, nutrition, light and humidity (Joshi, 2018;
Shaban, 2013). These factors must be conditioned to
obtain sprouts with a uniform height.
Amylase enzyme isolation process carried out on
150 grams of durian seed sprouts, at this stage
obtained an orange solution, which will then be
tested for its activity against variations on
temperature, pH and substrate concentration before
and after purification.
3.1 Crude Amylase Enzyme Activity
Test on Variation of Temperature
One of the main determinants of enzyme activity is
temperature. This is because enzymes are part of
proteins that are sensitive to extreme changes in
temperature (Mohanan & Satyanarayana, 2018). In
this study the temperature range used is 30 – 50
0
C
with a range of 5
0
C. The amount of crude amylase
enzyme activity obtained in accordance with Figure
2
Figure 2: Activity of Crude Enzyme on Variation of
Temperature.
Based on Figure 2 above, it can be seen that the
activity of crude amylase enzyme produced is
influenced by temperature. At temperature of 30-40
0
C the enzyme activity has increased and the
optimum temperature is seen at a temperature of 40
0
C with value of activity is 36.87 U/ml and will
further decrease its activity until a temperature of 50
0
C. Temparature value of the activity of enzyme is
also influenced by the source of the enzyme
obtained. Asrat et al 2018 has isolated enzyme
amylase from Aspergillus Niger FAB-211, the
optimum temperature of enzyme amilase is 45 0C.
Several studies have reported that the optimal
activity for the amylase enzyme is at temperature
40°C if the enzyme is isolated from H.
bacteriophora, A. suum and S. Litorallis and 50°C
for α -amylases from C. flavus, S. alluvius
ATCC 26074, L. kononenkoae and C. antarctica
CBS 667 (Wanderley et al., 2004).
3.2 Crude Amylase Enzyme Activity
Test on Variation of pH
Besides temperature, another factor that determines
the activity of an enzyme is pH. The crude amylase
enzyme activity test results obtained in this study are
shown in Figure 3 below.
Isolation and Determination of Amylase Enzyme Activity from Durian Seed Sprouts
415
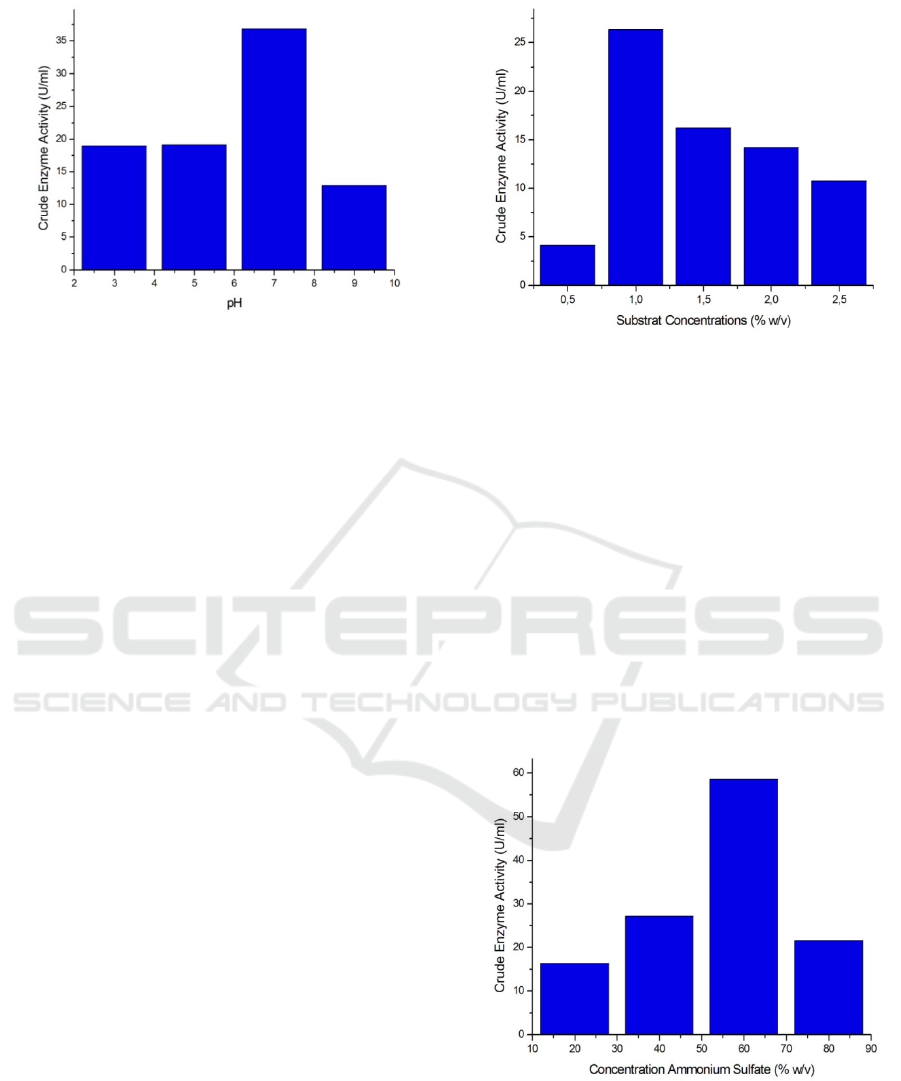
Figure 3: Activity of Crude Enzyme on Variation of pH.
Based on Figure 3 The following shows that at pH 3
and 5 the crude amylase enzyme activity shows no
difference, but when pH 7 shows the optimum
activity of crude enzyme amylase, the value of
activity ezymes is 36,86 U/ml and will decrease at
pH 9. Several studies have shown that the optimum
pH for several amylase enzymes is also in the pH
range of 6-6.5 (Asrat & Girma, 2018; Biazus et al.,
2009).
3.3 Crude Amylase Enzyme Activity
Test on Variation of Substrate
Concentrations
Comparison between enzymes and substrate
concentrations also needs to be considered because
if a comparison between enzymes and substrate is
appropriate a product with maximum hydrolysis
results will be obtained. In this study the results of
testing the activity of crude enzyme amylase on
substrate concentration are shown in Figure 4.
Based on Figure 4 below, it can be concluded
that the optimum substrate concentration that can be
hydrolyzed by the crude amylase enzyme isolated is
at a concentration starch solution 1%. This shows
that the ratio between enzymes and substrate is 1 ml
of enzyme with 0.5 ml of 1% starch substrate
obtained enzyme activity of 26.36 U/ml.
Figure 4: Activity of Crude Enzyme on Variation of
Substrat Concentration.
3.4 Crude Amylase Enzyme Activity
Test after Purification with
Ammonium Sulfate
The purity of enzymes is important because purer
enzymes will have better activity than before
purification. In this study crude enzyme amylase
was purified through a dialysis process that was
previously precipitated with ammonium sulfate at a
saturation level of 20% - 80% with range of 20%. At
this stage the enzyme activity measured was carried
out at the optimum temperature, pH and substrate
concentration that had been carried out previously.
The results obtained are shown in Figure 5 below.
Figure 5: Activity of Crude Enzyme on Variation of
Concentration Ammonium Sulfate.
Based on Figure 5 above it can be seen that after the
purification process the enzyme amylase activity has
increased than before the dialysis process. The
optimum activity of the amylase enzyme obtained at
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
416

60% ammonium sulfate concentration, the value of
activity enzyme is 58.59 U/ml. Based on this it can
be concluded that the purification process is one of
the important things that must be done to see the
activity of an enzyme.
4 CONCLUSIONS
Based on the research that has been done, it can be
concluded that durian seed sprouts can be used as a
source of amylase enzymes. Crude Activity The
amylase enzyme produced in this study before being
purified had optimum activity at a temperature of 40
0C, pH 7, and 1% substrate concentration obtained
activity values for each of 36.87 U/ml, 36.86 U/ml,
and 26.36 U/ml. The optimum conditions obtained
were then used to determine the crude activity of the
amylase enzyme after purification through dialysis
which was previously precipitated with ammonium
sulfate and obtained optimum results at a
concentration of 60% ammonium sulfate with
enzyme activity of 58.59 U/ml.
ACKNOWLEDGEMENT
The researcher would like to thank to Chancellor of
Universitas Sumatera Utara through the research
institution of Universitas Sumatera Utara for funding
this research with no contract
4167/UN5.1.R/PPM/2019. On April 2019
REFERENCES
Asrat, B., & Girma, A. (2018). Isolation, Production and
Characterization of Amylase Enzyme Using the
Isolate Aspergillus niger FAB-211. International
Journal of Biotechnology and Molecular Biology
Research, 9(2), 7–14.
Biazus, J. P. M., Souza, R. R. de, Márquez, J. E., Franco,
T. T., Santana, J. C. C., & Tambourgi, E. B. (2009).
Production and characterization of amylases from Zea
mays malt. Braz. Arch. Biol. Technol., 52(4), 991–
1000. https://doi.org/https://doi.org/10.1590/S1516-
89132009000400024
Chapman, J., Ismail, A. E., & Dinu, C. Z. (2018).
Industrial Applications of Enzymes: Recent Advances,
Techniques, and Outlooks. Catalysts, 8(6), 1–26.
https://doi.org/https://doi.org/10.3390/catal8060238
Joshi, R. (2018). Role of Enzymes in Seed Germination.
International Journal of Creative Research Thoughts,
6(2), 1481–1485.
Mohanan, N., & Satyanarayana, T. (2018). Amylases
Reference Module in Life Sciences. Elsevier Inc.
Naili, B., Sahnoun, M., Bejar, S., & Kammoun, R. (2016).
Optimization of submerged Aspergillus oryzae S2 α-
amylase production. Food Science and Biotechnology,
25(1), 185–192.
Shaban, M. (2013). Effect of Water and Temperature on
Seed Germination and Emergence as A Seed
Hydrothermal Time Model. International Journal of
Advanced Biological and Biomedical Research, 1(12),
1686–1691.
Simair, A. A., Qureshi, A. S., Khushk, I., Ali, C. H.,
Lashari, S., Bhutto, M. A., Mangrio, G. S., & Lu, C.
(2017). Production and Partial Characterization of α-
Amylase Enzyme from Bacillus sp. BCC 01-50 and
Potential Applications. BioMed Research
International, 2017, 1–9.
https://doi.org/https://doi.org/10.1155/2017/9173040
Sindhu, R., Binod, P., Madhavan, A., Beevi, U. S.,
Mathew, A. K., Abraham, A., Pandey, A., & Kumar,
V. (2017). Molecular improvements in microbial α-
amylases for enhanced stability and catalytic
efficiency. Bioresour Technol., 245, 1740–1748.
Wanderley, K. J., Torres, F. A. G., Moraes, L. M. P., &
Ulhoa, J. C. (2004). Biochemical characterization of α‐
amylase from the yeast Cryptococcus flavus. FEMS
Microbiology Letters, 231(2), 165–169.
https://doi.org/https://doi.org/10.1016/S0378-
1097(03)00955-8
Isolation and Determination of Amylase Enzyme Activity from Durian Seed Sprouts
417
