
A New Colorimetric Sensor Responding CN- Anion based on
Hydrazone Compound in Acetonitrile Solution
S. Suharman
1
and Siti Utari Rahayu
2
1
Department of Chemistry, Universitas Sumatera Utara, Jl. Dr. T. Mansyur No. 9, Medan, Indonesia
2
Department of Physics, Universitas Sumatera Utara, Jl. Dr. T. Mansyur No. 9, Medan, Indonesia
Keywords: Chemosensor, Colorimetric, Cyanide, Hydrazone, Vanillin.
Abstract: A new hydrazone compound 2-methoxy-6-((E)-phenyldiazenyl)-4-((E)-(2-phenylhydrazineylidene)
methyl)phenol (receptor R) based on vanillin has been synthesized and applied to cyanide anion detection in
acetonitrile solvent. Color of the receptor solution turned from yellow to purple in the presence of cyanide
anion and other anions like H
2
PO
4
-
, Br
-
, CN
-
, SO
4
2-
, Cl
-
, I
-
, CH
3
COO
-
and F
-
could not effect color of
receptor. Changing in the maximum wavelength of the receptor after addition of cyanide anion was
confirmed by UV-Vis spectrophotometer. In the presence of cyanide, wavelength of the receptor changed
from 347 nm to 510 nm. Detection limit of the receptor were also observed by UV-Vis spectrophotometer.
Based on the UV-Vis titration, the receptor detected cyanide anions with limit of detection 4mM in the
acetonitrile solvent.
1 INTRODUCTION
The anion waste produced from natural processes or
industries like gold mining, electroplating, metal and
pesticides industries cause health and environmental
problems (Ghanavati et al., 2014). Some anions
waste like cyanide have toxic properties and a lethal
effect (Mourzinaet al., 2003;Nezamzadeh-ejhieh and
Esmaeilian, 2012) even exposure to large amount of
cyanide through both Inhalation or oral exposure
cause death and systemic effect (respiratory,
cardiovascular, gastrointestinal, neurological,
haematological) on living organisms (Research
Triangle Institute, 1997). WHO has established level
of cyanide in drinking-water 0.5 mg/L (WHO,
2011). The toxic effect of cyanide can be described
from its ability to inhibit enzyme cytochrome
oxidase on step of oxidative phosphorylation of
respiration process (Gupta, 2009). Cyanide anion
can form complex phenomenon with a part of
enzymatic structure that can inhibit function and
cellular growth (Mekuto et al., 2016).
Severalmethods have been widely used to detect
the presence of anions waste in the environment
including potentiometric (Mourzinaet al.,
2003;Nezamzadeh-ejhieh & Esmaeilian, 2012;
Cuartero et al., 2013), ion chromatography (Silveira
et al., 2014) and electrochemical analysis (Pulkkaet
al., 2014). They are less practical methods that
sample preparation is required. In addition, the
analysis of anion by the methods require high cost
and a long time for analysis. Therefore we need
more practical method for detecting these anions.
Colorimetric chemosensor method, a method
developed for the detection of anions, is more
efficient method which is not require sample
preparation and the specific instrument.
Furthermore, analysis with this method is directly
observed by naked eye. It uses sensor compounds
called chemosensor which generally consists of two
parts: The binding site like -NH or -OH groups plays
as fragment that can interact with anions and
signaling subunit like chromophore groups is act as
a signal transducer (Martinez-Manez & Sancenon,
2003). Both binding site and signaling sub unit are
important part in the colorimetric sensor which gives
effect to its selectivity and sensitivity to anion.
Organic materials have been widely used as
chemosensor. Recently, researcher have been
reported the colorimetric anion based chalcone, azo
and hydrazone derivatives. Azo derivative was used
as sensor of H
2
PO
4
-
, F
-
and acetate anion with the
amine group as the binding site (Shao et al, 2009).
Hydrazone derivative was also used as sensor of
acetate anion by amine group on the synthesized
314
Suharman, S. and Rahayu, S.
A New Colorimetric Sensor Responding CN- Anion based on Hydrazone Compound in Acetonitrile Solution.
DOI: 10.5220/0010163400002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 314-318
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

compound as binding site that interact with the
acetate anion through hydrogen bonds (Qiao, 2009).
The color change of the synthesized compound
solution in DMSO solvent from yellow to green was
occurred after addition of acetate anion. In addition,
azo-hydrazone derivative such as 4-phenylazo-2-
hydroxy-benzaldehydephenylhydrazone compound
from salicylaldehyde was used as compound sensor
by the change of color from yellow to red after the
addition of acetate, F- and H
2
PO
4
-
anions (Li et al.,
2010).
In this study, we synthesized receptors 2-
methoxy-6-((E)-phenyldiazenyl)-4-((E)-(2-
phenylhydrazineylidene)methyl)phenol (receptor R).
We also present the solvent (solvatochromic) and
electron-withdrawing group like azo effect to UV-
Vis electronic absorption and sensitivity of receptors
R to cyanide anion.
2 EXPERIMENTAL SECTION
2.1 General
Vanillin, NaOH, methanol, acetic acid, H
2
SO
4
98% ,
ethanol, NaNO
2
, CHCl
3
, phenylhydrazine, DMSO,
acetonitrile, acetone, silica gel 60 (0.040-0.063 mm),
NaF, NaCl, NaI, NaBr, Na
2
CO
3
, Na
2
SO
4
, NaH
2
PO
4
,
CH
3
COONa, NaCN. All materialswere purchased
from Merck.Infrared spectra were recorded using
Shimadzu Prestige-21 FT-IR Spectrophotometer.
The spectra of
1
H-NMR and
13
C-NMR were
evaluated on JEOL JNM ECA-500, Mass spectra
were performed on Shimadzu QP-QP-5000 and
2010. Melting point was measured using uncorrected
Electrothermal-9100
2.2 Synthetic Procedure of 4-hydroxy-
3-methoxy-5-
(phenyldiazenyl)benzeldehyde
Solution I (aniline0.03 mol, water (7.5 mL) and
H2SO4 7.5 mL) was addedinto sodium nitrite
solution (0.03 mol, water 12 mL) and stirred for 1 h.
Solution II (NaOH 3.6 g in water 90 mL and vanillin
0.03 mol, 0-5
o
C)was dropwised into the solution I.
The solution was stirred for 3 at temperature 0-5
o
C.
The precipitate was filtered (Radchatawedchakoon
et al., 2014). Product was obtained as dark red solid
and yield 72%. IR cm-1: 3425 (O-H); 3062 (Csp
2
-
H), 2931 and 2864 (Csp
3
-H); 1681 (C=O); 1604
(C=C aromatic); 1458 (N=N); 1411(-CH
3
); 1280 (C-
N); 1141 and 1072 (C-O-C).
1
H-NMR (CDCl
3
):
δ/ppm 14.23 (O-H, s, 1H); 9.94 (CHO, s, 1H); 7.88
(m, 2H); 7.56-7.52 (m, 3H); 7.48 (s, 1H), 4.00
(OCH
3
, s, 3H). EI-MS (m/z): 256.
2.3 Synthetic Procedure of Receptor R
The compound of 4-hydroxy-3-methoxy-5-
(phenyldiazenyl)benzaldehyde (1mmol) in ethanol
(100 mL) was introduced into the base-round three
neck flask capacity 250 mL with a condenser.
Phenylhidrazine (1 mmol) and four drop of acetic
acid were dropwised into the solution. The solution
stirred and refluxed for 3 h. The precipitate was
filtered. Product was produced as dark brown solid,
yield 81%, m.p. 141-141.8
o
C. IR (cm
-1
): 3425 (O-
H); 3309 (N-H); 3055 (Csp
2
-H); 2931 and 2854
(Csp
3
-H); 1597 (C=N); 1496 (N=N); 1265 (C-N);
1149 and 1072 (C-O-C).
1
H-NMR (DMSO-
d6;δ/ppm): 13.66 (NH, s, 1H); 7.78 (aromatic, d, J =
1.95 Hz, 2H); 7.74 (CH=N, s, 1H; 7.63 (OH, s, 1H);
7.56-7.48 (m, 5H); 7.3-7.28 (m, 2H); 7.14 (s, 2H);
6.88 ( s, 1H); 4.03 (-OCH
3
, s, 3H).
13
C-NMR
(DMSO-d6;δ/ppm): 149.9; 149.6; 145.4; 136.8;
131.4; 129.7; 129.6; 129.5; 127.0; 123.6; 122.5;
122.2; 120.3; 112.9; 110.5; 56.6. EI-MS (m/z): 346
(M+).
2.4 Solvatochromic Study of Receptor
R
Solvatochromic studies of receptors R were used
various solvent such as ethanol, DMSO, acetonitrile
and acetone. 4.0 mg of Receptor was dissolved in
solvents. The color change and the UV-Vis
absorptions were recorded.
2.5 Selectivity Study of Receptor R
Selectivity studies of receptor R were carried out in
acetonitrile solution at concentration level 2 x 10
-5
M. The solution of receptor was added by 10 µL of
NaF, NaCl, NaBr, NaI, NaCN, Na
2
SO
4
, Na
2
CO
3
,
CH
3
COONa and NaH
2
PO
4
solution, respectively.
The color change and the UV-Vis absorption were
recorded.
2.6 UV-Vis Titration of Receptor R
with CN
-
The titration of UV-Vis was carried out in
acetonitrile solution at concentration level of 5 x 10
-5
M for receptor R and (1-10) x 10
-2
and (1-10) x 10
-3
M for CN
-
. The solution of receptor was added by 50
µL of CN
-
solution. The UV-Vis absorptions were
A New Colorimetric Sensor Responding CN- Anion based on Hydrazone Compound in Acetonitrile Solution
315
recorded by spectrophotometer UV-Vis at 200-700
nm.
3 RESULT AND DISCUSSION
The receptor R was synthesized by condensation
reaction between 1 mmol of 4-hydroxy-3-methoxy-
5-(phenyldiazenyl) benzaldehyde and I mmol of
Phenylhidrazinein 100 mL ethanol solvent (Fig. 1).
The chemical structure of receptor Rwere confirmed
byGC-MS, FT-IR,
13
C-NMR and
1
H-NMR.
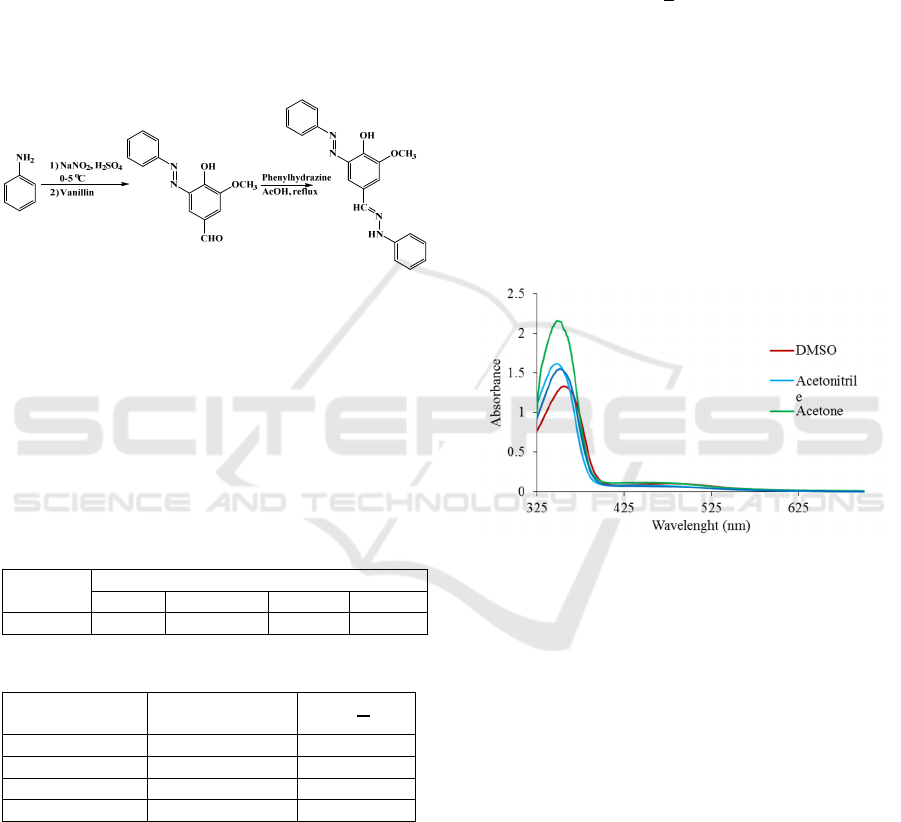
Figure 1: Synthesis of receptor R.
3.1 Solvatochromic Study
Solvatochromic studies of receptor R carried out
with variety of protic and non-protic solvents. Table
1 shows the maximum wavelength of receptor R in
various solvents and Fig. 2 shows solvatochromic
absorbance spectra of receptor.
Table 1: λmax values of receptor 1, 2 and 3 in various
solvents.
Receptor λmax (nm)
DMSO acetonitrile Acetone Ethanol
R 355 347 348 351
Table 2: ET(30) and normalized EN/T value.
Solvent ET (30)
(Kcal mol-1)
E
N
T
Ethanol 51.9 0.654
DMSO 45.1 0.444
Acetonitrile 45.6 0.460
Acetone 42.2 0.355
Based on Table 1 showed that polar solvent such
as DMSO and ethanol caused bathochromic shift
compared to less polar solvent such as acetonitrile
and acetone. Solvent polarity causes the different of
maximum wavelength of receptor R. Based on the
UV-Vis electronic absorption, maximum wavelength
of receptor in DMSO solvent was 355 nm, while in
acetonitrile solvent was 390 nm. Hypsochromic shift
occurred at acetone (λmax 348 nm and acetonitrile
(λmax 347 nm) solvent. The difference of solvent
polarity affects electronic transition energy of the
ground state to the excited state. More polar solvents
such as DMSO stabilizes the electron in the excited
state (π*) that causes the transition energy becomes
smaller so maximum wavelength will be greater. In
addition, protic (DMSO) and aprotic (ethanol)
solvent affect maximum wavelength of receptor.
Based on normalized
E
N
T
and ET (30) value, ethanol
solvent is more polar than solvent DMSO (see Table
2). However, maximum wavelength of receptor in
ethanol solvent is smaller than the DMSO solvent.
Transition energy of the ground state to the excited
stateis influenced by the ability of the solvent to
form hydrogen bond with receptor. Protic solvent
such as ethanol can form hydrogen bonds with the
receptor that causes free electrons in the ground state
is more stabilized than the excited state. Therefore,
the energy transition will be greater and maximum
wavelength will be smaller (Reichardt, 1994).
Figure 2: Solvatochromic UV-Vis spectra of receptor R.
Figure 2 shows the UV-Vis spectra of receptors
R. Table 1 and Fig. 2 shows that the substituent at
aromatic ring affect the λmax value between
receptor R. Electron-withdrawing group such as azo
group increases delocalization of electron which it
caused the electron more conjugated and
bathochromic shift (Huang et al., 2012). It is attested
by the presences of azo group at receptor increased
the intensity of color change and wavelength shift of
receptor.
3.2 Selectivity Study of Receptor R
The selectivity of receptor was evaluated by adding
anions likeH
2
PO
4
-
, CO
3
2-
, Br
-
, CN
-
, SO
4
2-
, Cl
-
, I
-
,
CH
3
COO
-
and F
-
to acetonitrile solution of receptors
R. It was observed that the addition of CO
3
2-
and
CN
-
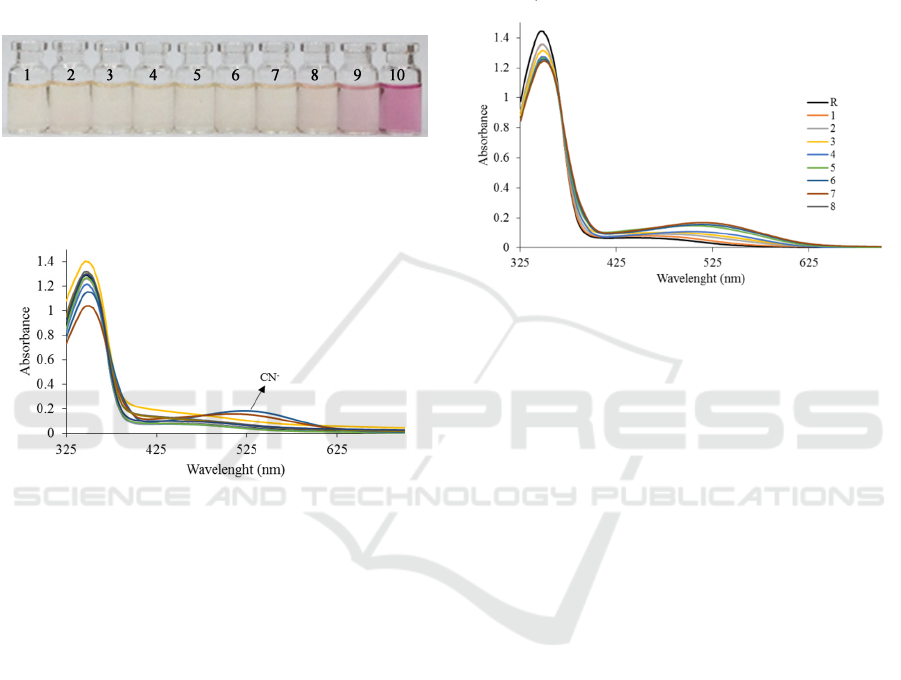
anions to receptor solution, the receptor solution
color changed from bright yellow to purple (Fig. 3).
Other anions such as H
2
PO
4
-
, Br
-
, SO
4
2-
, Cl
-
, I
-
,
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
316
CH
3
COO
-
,F
-
did not provide color change of receptor
solution. In addition, the absorbance spectra were
determined by UV-Vis spectrophotometer. Out of all
anions examined, the presence of CN
-
in solution
appeared new band at 510 nm. New band appeared
at 522 nm after addition CO
3
2-
but the absorbance
intensity of CN
-
were greater than CO
3
2-
(Fig. 4).
Based on the study, we concluded that the receptor
could be used as a colorimetric sensor for CN
-
in
acetonitrile solvent.
Figure 3: The color change of receptor 3 in CH
3
CN after
addition of anions: 1) Receptor, 2) F
-
, 3) Cl
-
, 4) Br
-
, 5) I
-
,
6) H
2
PO
4
-
, 7) CH
3
COO
-
, 8) SO
4
2-
, 9) CO
3
2-
, 10) CN
-
.
Figure 4: Titration of UV-Vis of receptor R with Cyanide.
The color change caused by the presence of CN
-
in solution proves that there is an interaction
between receptor and CN
-
. We suggested three
possible mechanisms. The first interaction is the
hydrogen bond between CN
-
with O-H or N-H
group. The second interaction is deprotonation of H
atom at hydroxyl or amine group (Mondal et al.,
2018). The last possible mechanism is
chomodisimeter: CN
-
, which is a strong nucleophile,
attacks C=N to form a new C-C bond (Cao & Wang,
2013).
3.3 UV-Vis Titration of Receptor R
with CN
-
UV-Vis titration receptors R were carried out in
acetonitrile solution at a concentration level 2 x 10
-5
M. the ability of anion to form bond with receptor R
was evaluated by adding solution of sodium cyanide
salt. The addition of CN
-
anion also affected UV-Vis
spectra of receptor 3 (Fig. 5). The addition of CN-
anion to receptor caused absorbance at 347 nm
decreased gradually and new band appeared at 510
nm. In addition, the change of color from yellow to
purple occurred after addition of cyanide anion to
receptor (Fig. 3). The color change and wavelength
shift after the addition of CN
-
anion indicated that
the receptors reacted with CN
-
anion. We concluded
that receptor could be used to cyanide detection by
naked eye.
Figure 5: UV-Vis titration of R with Cyanide.
Receptor R have two active sites part i.e. amine
and hydroxyl groups. However, the ability of amine
group to form hydrogen bond with cyanide anion
was better than hydroxyl group. The presence of azo
group on the receptor caused the proton on the
amine group is more acid than the hydroxyl group
(Shang and Xu, 2009). Thus, Interaction occurs
between -NH at receptor with CN
-
anion. The
presence of chromophore group such as azo group
affect the sensitivity of the receptor to the CN
-
anion. It is attested by detection limit of receptor.
Based on Fig. 5, the color change at receptor
occurred after the addition of 4 mM of CN
-
anion.
4 CONCLUSIONS
Receptor R show the solvatochromic properties in
protic and aprotic solvents. In aprotic solvents,
bathochromic shift of receptors occur in more polar
solvents, while protic solvent such as ethanol can
form H-bonds with binding site of receptor that can
stabilize the electron in the ground state. It causes
the λmax value of receptor in ethanol is smaller than
the DMSO. The presence of electron withdrawing-
group like azo group can increase sensitivity of
receptor to cyanide anion. It is evidenced by the
limit of detection of receptor was 4 mM.
A New Colorimetric Sensor Responding CN- Anion based on Hydrazone Compound in Acetonitrile Solution
317

ACKNOWLEDGEMENTS
This research was funded by Universitas Sumatera
Utara with research implementation contract number
in fiscal year 2019 Number:
4167/UN5.1.R/PPM/2019 dated April 1
st
,2019.
REFERENCES
Cao, J., & Wang, X. (2013). An investigation of the
deprotonation of hydrazone-based receptors on
interaction with anion : develop a colorimetric system
distinguishing cyanide from anions. Tetrahedron,
69(48), 10267–10271.
https://doi.org/10.1016/j.tet.2013.10.030
Cuartero, M., García, M. S., & Ortu, J. A. (2013).
Differential dynamic potentiometric responses
obtained with anion-selective electrodes for
perchlorate , thiocyanate , iodide , nitrate , sulfate ,
picrate and bis ( trifluoromethylsulfonyl ) imide.
Electrochimica Acta, 93, 272–278.
https://doi.org/10.1016/j.electacta.2013.01.101
Ghanavati, M., Azad, R. R., & Mousavi, S. A. (2014).
Amperometric inhibition biosensor for the
determination of cyanide. Sensors & Actuators: B.
Chemical, 190, 858–864.
https://doi.org/10.1016/j.snb.2013.09.055
Gupta, R. C. (2009). Handbook of Toxicology of Chemical
Warfare Agents. Academic Press.
Huang, X., Gu, X., Zhang, G., & Zhang, D. (2012). A
highly selective fluorescence turn-on detection of
cyanide based on the aggregation of
tetraphenylethylene molecules induced by chemical
reaction. Chem. Commun., 48(100), 12195–12197.
https://doi.org/doi.org/10.1039/c2cc37094h
Li, Y., Li, J., Lin, H., Shao, J., Cai, Z.-S., & Lin, H.
(2010). A novel colorimetric receptor responding
AcO− anions based on an azo derivative in DMSO and
DMSO/water solution. Journal of Luminescence,
130(3), 466–472.
https://doi.org/10.1016/j.jlumin.2009.10.015
Martinez-Manez, R., & Sancenon, F. (2003). Fluorogenic
and Chromogenic Chemosensors and Reagents for
Anions. Chem. Rev., 103, 4419–4476.
Mekuto, L., Ntwampe, S. K. O., & Akcil, A. (2016). An
integrated biological approach for treatment of
cyanidation wastewater. Science of the Total
Environment, 571, 711–720.
https://doi.org/10.1016/j.scitotenv.2016.07.040
Mondal, J., Manna, A. K., & Patra, G. K. (2018). Highly
selective hydrazone based reversible colorimetric
chemosensors for expeditious detection of CN − in
aqueous media. Inorganica Chimica Acta, 474, 22–29.
https://doi.org/10.1016/j.ica.2018.01.013
Mourzina, Y. G., Ermolenko, Y. E., Yoshinobu, T., &
Vlasov, Y. (2003). Anion-selective light-addressable
potentiometric sensors ( LAPS ) for the determination
of nitrate and sulphate ions. Sensors and Actuators B,
91, 32–38. https://doi.org/10.1016/S0925-
4005(03)00063-7
Nezamzadeh-ejhieh, A., & Esmaeilian, A. (2012).
Microporous and Mesoporous Materials Application
of surfactant modified zeolite carbon paste electrode (
SMZ-CPE ) towards potentiometric determination of
sulfate. Microporous and Mesoporous Materials,
147(1), 302–309.
https://doi.org/10.1016/j.micromeso.2011.06.026
Pulkka, S., Martikainen, M., Bhatnagar, A., & Sillanpää,
M. (2014). Electrochemical methods for the removal
of anionic contaminants from water – A review.
Separation and Purification Technology, 132, 252–
271. https://doi.org/10.1016/j.seppur.2014.05.021
Qiao, Y.-H., Lin, H., Shao, J., & Lin, H.-K. (2009). A
highly selective naked-eye colorimetric sensor for
acetate ion based on 1,10-phenanthroline-2,9-
dicarboxyaldehyde-di-(p-substitutedphenyl-
hydrazone). Spectrochimica Acta. Part A, Molecular
and Biomolecular Spectroscopy, 72(2), 378–381.
https://doi.org/10.1016/j.saa.2008.10.007
Radchatawedchakoon, W., Sangsuwan, W., Kruanetr, S.,
& Sakee, U. (2014). Synthesis and evaluation of
simple naked-eye colorimetric chemosensors for
anions based on azo dye-thiosemicarbazones.
Spectrochimica Acta. Part A, Molecular and
Biomolecular Spectroscopy, 121, 306–312.
https://doi.org/10.1016/j.saa.2013.10.086
Reichardt, C. (1994). Solvatochromic Dyes as Solvent
Polarity Indicators. Chemical Reviews, 94(8), 2319–
2358. https://doi.org/10.1021/cr00032a005
Research Triangle Institute. (1997). Toxicological Profile
for Cyanide. In U.S Dept of Health and Human
Services (Issue November). U.S. Departement of
Health and Human Services.
https://doi.org/http://dx.doi.org/10.1155/2013/286524
Shang, X., & Xu, X. (2009). The anion recognition
properties of hydrazone derivatives containing
anthracene. BioSystems, 96(2), 165–171.
https://doi.org/10.1016/j.biosystems.2009.01.003
Shao, J., Lin, H., & Lin, H. (2009). A novel chromo- and
fluorogenic dual responding H2PO4− receptor based
on an azo derivative. Dyes and Pigments, 80(2), 259–
263. https://doi.org/10.1016/j.dyepig.2008.07.012
Silveira, E. L. C., de Caland, L. B., & Tubino, M. (2014).
Simultaneous quantitative analysis of the acetate,
formate, chloride, phosphate and sulfate anions in
biodiesel by ion chromatography. Fuel, 124, 97–101.
https://doi.org/10.1016/j.fuel.2014.01.095
WHO. (2011). Guidelines for Drinking-water Quality
(fourth). WHO Press.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
318
