
Effect of Variation HCl Concentration on Natural Zeolite
Dealumination to The Content of Liquid Smoke Compounds by
Hydrodeoxygenation Process
Saharman Gea
1*
, Andriayani
1
, Agus Haryono
3
, Abdul Malik
1
, Reka Mustika Sari
1
, Junifa Layla
Sihombing
1,2
, Ahmad Nasir Pulungan
1,2
, Rachmad Fauzi
1
and Boy Attaurrazaq
1
1
Department of Chemistry, Universitas Sumatera Utara, Jl. Bioteknologi No. 1, Medan, Indonesia
2
Department of Chemistry, Universitas Negeri Medan, Jl. Willem Iskandar Psr. V Medan, Indonesia
3
Research Centre for Chemistry, Indonesian Institute of Sciences, Banten, Indonesia
{junifalaylasihombing, nasirpl}@unimed.ac.id, {rachmadfauzi8888, boyattaurrazaq37}@gmail.com
Keywords: Dealumination, Hydrodeoxygenation Process, Liquid Smoke, Sarulla Natural Zeolite.
Abstract: Recently, for its direct use as a renewable energy resource of pyrolysis biomass, liquid smoke has received
increased attention. Unfornatunately, the composition of liquid smoke is very complex that cause to obvious
changes in chemical properties of the liquid smoke. Therefore, the hydrodeoxygenation method required to
handle this issue. The Objective of this research was the hydrodeoxygenation of liquid smoke to remove
oxygenated compounds with decreased concentration of ketones and increased phenol concentration using
sarulla natural zeolite dialuminated with 3M, 5M and 7M HCl concentrations at 90°C in the H
2
atmosphere.
Characterization of hydrodeoxygenation product was analyzed content by gas chromatography-mass
spectrometry (GC-MS). The results showed a decrease in phenol content and a rise in carbonyl at 7 M HCl
dealumination while 5 M HCl dealumination increased phenol content and reduced carbonyl compounds.
Treatment with concentration of 5 M HCl results in a better content of liquid smoke.
1 INTRODUCTION
Liquid smoke, a by-product of the charcoal industry,
is high economic in aspects of its discharge into the
atmosphere. Liquid smoke is derived from dew
condensation due to the decay of organic compounds
during pyrolysis. The quantity of liquid pyrolysis
smoke is 90.75% phenol compound, 3.71 %
carbonyl and 1.81% alcohol, an antimicrobial as a
meat preservative (Hadanu & Apituley, 2016).
Liquid smoke produced from pyrolysis has a
higher water and oxygen content and a lower heating
value than fossil fuels, which can be achieved by
upgrading, i.e. hydrodeoxygenation (HDO)
(Bulushev & Ross, 2011). HDO method can reduce
the water and oxygen content and increase the
heating value of biomass (S. P. Zhang et al., 2003).
A catalyst is necessary in the HDO process to
reduce the oxygenate compound content in liquid
smoke. Several studies have used catalysts Pd/C,
Ru/C and Pt/C (300°C, 3 MPa H
2
, 60 minutes) (Oh
et al., 2016) stored (23°C, 20% RH) for 12 weeks)
almost no change in color and physicochemical
properties. The water content changed from 1.3% to
3.4% is still in the dry category (<6%), the acidity
was reduced from 70.2 to 71 mg-KOH/g and HHV
from 762 Da to 867 Da. Likewise for bio oil after
HDO with Pt/Al
2
O
3
, Ni/HY, Pd/C catalyst (260°C,
70 Bar, 2 hours), after 140 hours there was no
change in content except 0.8% to 2.9% aldehyde and
30% phenol to 27% (Alvarez-Galvan et al., 2019;
Liu et al., 2019; Mortensen et al., 2011).
In the other hand, with high temperatures (above
300°C) and high H
2
pressure (2-10 MPa), the
catalyst often becomes inactive due mainly to coke
and water-induced structural changes (Mortensen et
al., 2011). In terms of costs as well as unnecessary
protection. Lercher and Jones show that
cyclohexane-forming phenol hydrodeoxygenation
can be performed at relatively low temperatures
using bi-functional metallic acid catalysts (Mäki-
Arvela & Murzin, 2017; Mo et al., 2018). Therefore
research has carried out the HDO process with
ethanol solvents which act as hydrogen donors and
Gea, S., Andriayani, ., Haryono, A., Malik, A., Mustika Sari, R., Layla Sihombing, J., Nasir Pulungan, A., Fauzi, R. and Attaurrazaq, B.
Effect of Variation HCl Concentration on Natural Zeolite Dealumination to the Content of Liquid Smoke Compounds by Hydrodeoxygenation Process.
DOI: 10.5220/0010152300002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 287-291
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
287
the catalyst used was natural zeolite. This study was
to determine the composition of compounds from
liquid smoke using natural zeolite as a catalyst
without the addition of metals.
2 RESEARCH METHODS
2.1 Materials
In this research using commercial liquid smoke,
natural zeolite purchased at bratachem. Ethanol
(p.a), hydrogen gas and nitrogen gas.
2.2 Activation Natural Zeolite
Zeolite was sieved with the size in 100 mesh and
then washed with distilled water for 24 hours then
filtered and dried at 110°C. The zeolite activation
was carried out by varying the HCl concentrations of
3M, 5M and 7M for 60 minutes at 90°C. After that,
filtered and washed to neutral pH and then dried.
Activated zeolite was calcined at 500°C for 1 hour
while running nitrogen gas 10 mL/min (Sihombing
et al., 2018).
2.3 Pretreatment of Liquid Smoke
Conventional liquid smoke was distilled to separate
water content. Distillation was carried out at 90°C.
After the liquid smoke was water-free, it was
prepared to be used for the cycle of
hydrodeoxygenation.
2.4 Hydrodeoxygenation of Liquid
Smoke
The hydrodeoxygenation process was carried out by
adding natural zeolite to liquid smoke at a ratio of
1% (w / w), then 96% ethanol was added, and
refluxed at 90°C while flowing 10 mL/min hydrogen
gas for 5 hours. After that, GC-MS measured the
components of the compounds.
3 RESULTS AND DISCUSSION
3.1 Identification of Components of
Liquid Smoke Compounds
GC-MS characterization was carried out to
determine the content of compounds in bio-oil. In
accordance with the main purpose of the research
was to vary the catalyst with different concentrations
can affect component compounds in liquid smoke,
i.e increase the content of phenols and reduce the
content of oxygenate compounds. GC-MS results
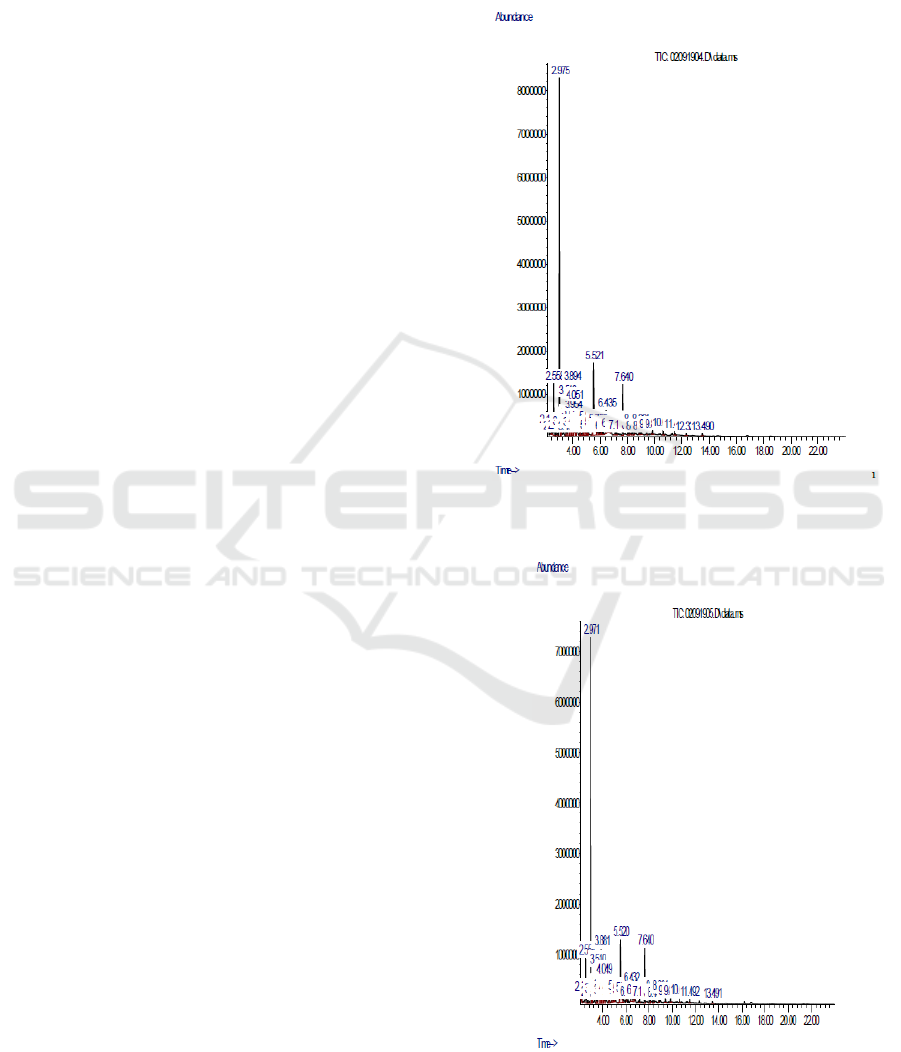
can be seen in Fig. 1-3.
Figure 1: GC-MS chromatogram analysis of liquid smoke
in 3M HCl Zeolite.
Figure 2: GC-MS chromatogram analysis of liquid smoke
in 5M HCl Zeolite.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
288
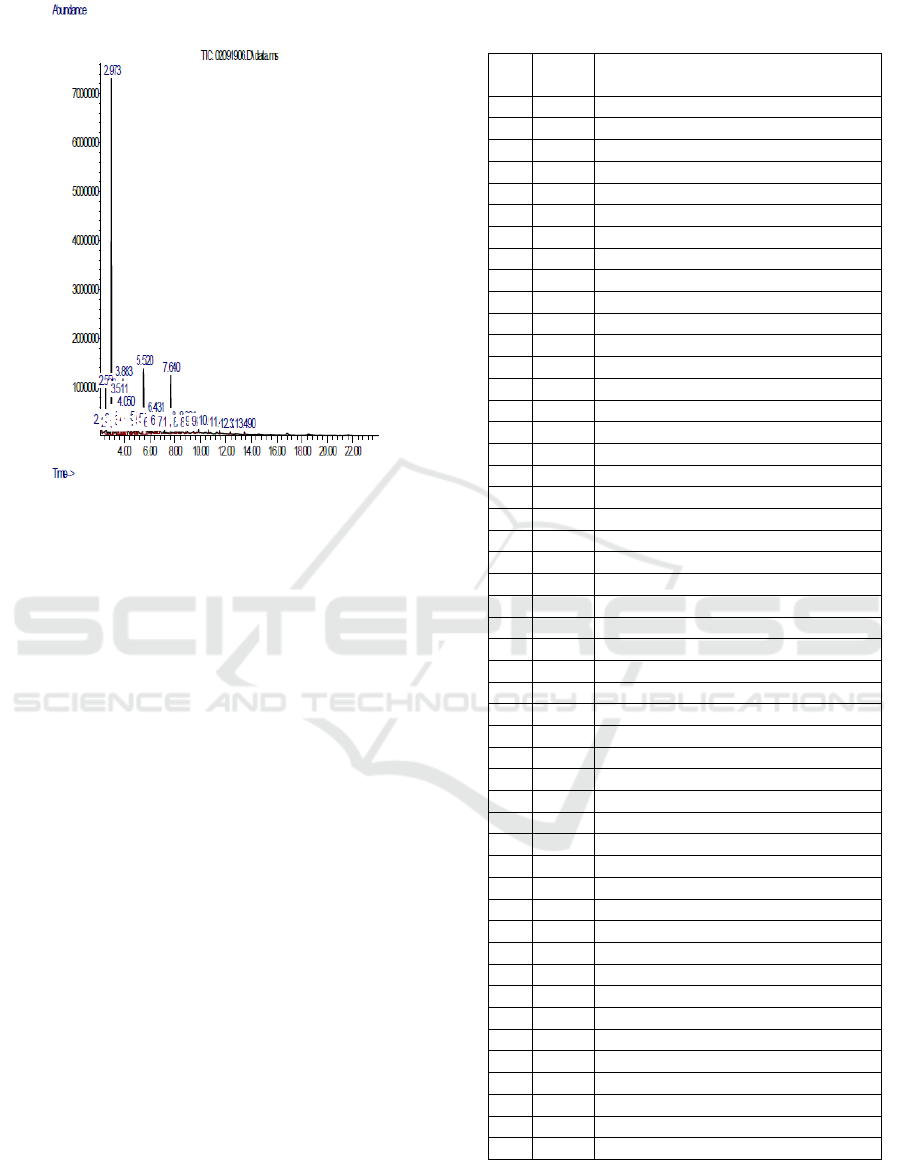
Figure 3: GC-MS chromatogram analysis of liquid smoke
in 7M HCl Zeolite.
There was more than 30 types of compound
components in liquid smoke which are shown in the
Table 1-3.
Table 1: Characterization GC-MS of liquid smoke in 3M
HCl Zeolite.
No
%
Area
Compounds
1 0.28 1,2-Ethanediol
2 0.17 Furan,tetrah
y
dro-2-meth
y
l
3 5.50 Furanone,dihydro
4 0.53 Furanone,5-methyl
5 35.71 Phenol
6 0.81 Phen
y
l alcohol
70.29 2-
p
i
p
eridinone
8 3.28 1,2-c
y
clo
p
entanedion
9 2.23 Pentanoic aci
d
10 1.97 Ethyl,4-hydroxybutanoate
11 0.63 Pentanoic acid,4-oxo,eth
y
l este
r
12 6.05 2-furanmethanol,tetrah
y
dro
13 2.74 Phenol,4-meth
y
l
14 2.64 Tetrahydrofurfurylaceate
15 0.85 Phenol,2-methoxy
16 0.39 3,hydroxy-2-methyl
17 079 2-c
y
clo
p
enten-1-one-3-eth
y
l
18 0.44 3-meth
y
lmor
p
holine
19 0.62 Dih
y
dro-citronella
20 1.13 2-furanone,5-methyl
21 0.38 Methyl este
r
22 1.08 2,2-dimeth
y
,1-one-2-silac
y
clo
23 0.45 Eth
y
l,5-oxohexanone
24 0.71 5-eth
y
ldeh
y
dro
25 13.66 1,2-
b
enzenediol
26 1.46 Cyclopropanecarboxamide
27 0.36 Phenol-d6
28 0.27 Tertrameth
y
l
g
uani
d
29 0.45 2-meth
y
l-3-isothiazolone
30 0.28 2-
p
enten,3-eth
y
l-2-meth
y
l
31 0.32 Phenol,3,4-dimethoxy
32 2.13 3-methyl-1-hexene
33 0.24 3-isobuth
y
ldih
y
dro
py
razin
34 0.67 7,7-dimeth
y
lbic
y
clo
35 0.39 2-n-
p
ro
py
lthiac
y
clohexane
36 0.42 Cyclohexane,ethyl
37 3.95 Phenol,2,6-dimethoxy
38 0.48 1-methoxy-2,6,6-trymethyl
39 0.32 3-
b
uten-2-ol,2-meth
y
l
40 1.00 Benzaldehide,3-h
y
drox
y
41 0.26 Phos
p
honic aci
d
42 0.73 Benzoic acid,4-hydroxy
43 0.42 Methylparaben
44 0.51 Ethanone
45 0.47 7,8-dimeth
y
lbenzoc
y
cloocetene
46 0.57 2,4-dih
y
drox
y
,3-methox
y
47 0.39 Benzaldehyde,4-hydroxy
48 0.24 Ethanone
49 0.24 Aspidinol
Effect of Variation HCl Concentration on Natural Zeolite Dealumination to the Content of Liquid Smoke Compounds by
Hydrodeoxygenation Process
289

Table 2: Characterization GC-MS of liquid smoke in 5M
HCl Zeolite.
No
%
Area
Compunds
1 0.27 Pro
p
anoic aci
d
2 0.63 2-
b
utanone
3 5.37 Furanone-dihydro
4 0.52 Furanone,5-methyl
5 40.02 Phenol
6 0.80 Furanone,3-meth
y
l
7 0.35 2-
p
i
p
eridinone
8 3.45 1,2-c
y
clo
p
entenedione
9 2.26 Pentanoic acid,4-oxo
10 2.08 Ethyl,4-hydroxybutanoate
11 0.55 Pentanoic acid,4-oxo eth
y
l este
r
12 6.09 2-furanmethanol,tetrah
y
dro
13 2.92 Phenol,4-meth
y
l
14 2.31 2-furanmethanol,tetrahidro-acet
15 0.93 Phenol,2-methoxy
16 0.31 3-hydroxy,2=methyl
17 076 2-c
y
clo
p
entene,1-one-3-eth
y
l
18 0.51 2-
p
ro
p
en
y
l,2-eth
y
lbutanone
19 0.83 2-furanone-5-meth
y
l
20 0.50 Ethane,1,1-diethoxy
21 0.69 2,2-dimethyl-1-oxa
22 0.52 2-furanone,dih
y
dro-5-
p
ent
y
l
23 12.87 1,2-
b
enzenediol
24 1.12 2-
p
ro
p
anoic acid,2-meth
y
l-eth
y
l
25 0.13 3-cis-methoxy-5-cus-methyl
26 2.23 Butanoid acid,butyl este
r
27 0.67 2,3-dihydroxy-acetophenone
28 0.39 C
y
clohexane eth
y
l
29 4.64 Phenol,2,6-dimethox
y
30 0.54 2,6-dimeth
y
l-4-oxa
31 0.23 1-hydroxy,2-
p
entanone
32 1.02 Benzaldehide,3-hydroxy
33 0.25 C
y
clo
p
entaneacetic aci
d
34 0.76 Benzoic acid,4-h
y
drox
y
35 0.51 Ethanone
36 0.62 2,3,5-trimethoxytoluene
37 0.48 Ethanone
38 0.44 Benzaldehide,4-hydroxy
39 0.42 As
p
inidiol
Table 3: Characterization GC-MS of liquid smoke in 7M
HCl Zeolite.
No
%
Area
Compounds
10.28 Pro
p
anoic aci
d
20.47 2-et
y
hlbutanal
3 5.31 2-furanone,dihydro
4 0.56 Furanone,5-methyl
5 36.57 Phenol
6 0.83 Phenol
70.39 2-
p
i
p
eridinone
8 3.38 1,2-c
y
clo
p
entanedione
9 2.29 Pentanoic aci
d
10 1.96 Ethyl,4-hydroxybutanoate
11 0.52 Pentanoic acid,4-oxo,eth
y
l
12 5.98 Tetrah
y
drofurfurilalkohol
13 2.66 Phenol,4-meth
y
l
14 2.14 2-furanmethanol
15 0.94 Phenol,2-methoxy
16 0.41 Maltol
17 0.85 2-c
y
clo
p
enten,1-one
18 0.75 4-acet
y
lbutric aci
d
19 1.11 Furanone,5-meth
y
l
20 0.65 Methylester of,4,4-dimethox
y
21 0.99 Methyl-2,3,4-triomethyl
22 0.73 Gamma,hexalactone
23 13.15 1,2-
b
enzenediol
24 1.40 2-
p
ro
p
anoic aci
d
25 0.52 N-methylthiazolone
26 0.39 Methyltriacelatone
27 2.22 Butanoic aci
d
28 0.65 1,4-
b
enzenediol
29 1.25 7,7-dimeth
y
lbic
y
clo
30 0.48 3,6-dih
y
dro-6,6-dimeth
y
l
31 4.55 Phenol,2,6-dimethoxy
32 0.51 Naphtalenone,octahydro
33 0.20 1-h
y
drox
y
-2-
p
entanone
34 0.94 Vanilin
35 0.28 C
y
clo
p
entaneacetic aci
d
36 0.75 Benzoic acid,4-hydroxy
37 0.40 Methylparaben
38 0.41 Ethanone
39 0.54 2,3,5-trimethox
y
toulene
40 0.51 Benzamide,dieth
y
l,4-h
y
drox
y
41 0.45 Benzaldeh
y
de,4-h
y
drox
y
42 0.28 Ethanone
43 0.34 Ethylisovanillymandelate
The high peak indicates a compound most
contained in bio-oil, namely phenol compounds in
the form of syringol and guaiacol. It can be seen
from the three tables above that phenol compounds
and oxygenate compounds are the main components
of bio-oil. Relative to other products, the syringol
compound has the highest and most important
appearance for all bio-oils. There is also a significant
percentage of guaiacol after syringol relative to other
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
290

compounds. Both of these compounds originate
from lignin cracking, while furan, acetate, and
aromatic compounds are derived from degradation
of cellulose and hemicellulose (C. Zhang et al.,
2016).
It appears that phenol compounds, both syringol
and guaiacol, have increased concentrations in this
case characterized by the percentage of the area on
the GC-MS chromatogram. It can be concluded that
the HDO reaction in bio-oil takes place which is
characterized by a decrease in the concentration of
oxygenate compounds such as ketones and an
increase in alcohol concentration in this case
represented by phenols.
But there was a decline in phenol levels at 7 M
zeolite concentration. The possibility of zeolite with
a concentration of 7M has not been able to be a good
catalyst in the HDO process.
4 CONCLUSIONS
Generally, the results showed a decrease in phenol
content and a rise in carbonyl at 7 M HCl
dealumination while 5 M HCl dealumination
increased phenol content and reduced carbonyl
compounds. Treatment with concentration of 5 M
HCl results in a better content of liquid smoke.
ACKNOWLEDGEMENTS
The authors would like to thanks to Universitas
Sumatera utara by TALENTA 2019 scheme.
REFERENCES
Alvarez-Galvan, M. C., Campos-Martin, J. M., & Fierro,
J. L. G. (2019). Transition metal phosphides for the
catalytic hydrodeoxygenation of waste oils into green
diesel. In Catalysts.
https://doi.org/10.3390/catal9030293
Bulushev, D. A., & Ross, J. R. H. (2011). Catalysis for
conversion of biomass to fuels via pyrolysis and
gasification: A review. Catalysis Today.
https://doi.org/10.1016/j.cattod.2011.02.005
Hadanu, R., & Apituley, D. A. N. (2016). Volatile
Compounds Detected in Coconut Shell Liquid Smoke
through Pyrolysis at a Fractioning Temperature of
350-420 C. Makara Journal of Science.
https://doi.org/10.7454/mss.v20i3.6239
Liu, M., Yi, Y., Wang, L., Guo, H., & Bogaerts, A.
(2019). Hydrogenation of carbon dioxide to value-
added chemicals by heterogeneous catalysis and
plasma catalysis. Catalysts.
https://doi.org/10.3390/catal9030275
Mäki-Arvela, P., & Murzin, D. Y. (2017).
Hydrodeoxygenation of lignin-derived phenols: From
fundamental studies towards industrial applications. In
Catalysts. https://doi.org/10.3390/catal7090265
Mo, L., Yu, W., Cai, H., Lou, H., & Zheng, X. (2018).
Hydrodeoxygenation of bio-derived phenol to
cyclohexane fuel catalyzed by bifunctional
mesoporous organic-inorganic hybrids. Frontiers in
Chemistry. https://doi.org/10.3389/fchem.2018.00216
Mortensen, P. M., Grunwaldt, J. D., Jensen, P. A.,
Knudsen, K. G., & Jensen, A. D. (2011). A review of
catalytic upgrading of bio-oil to engine fuels. In
Applied Catalysis A: General.
https://doi.org/10.1016/j.apcata.2011.08.046
Sihombing, J. L., Gea, S., Pulungan, A. N., Agusnar, H.,
Wirjosentono, B., & Hutapea, Y. A. (2018). The
characterization of Sarulla natural zeolite crystal and
its morphological structure. AIP Conference
Proceedings.
Zhang, C., Qi, J., Xing, J., Tang, S. F., Song, L., Sun, Y.,
Zhang, C., Xin, H., & Li, X. (2016). An investigation
on the aqueous-phase hydrodeoxygenation of various
methoxy-substituted lignin monomers on Pd/C and
HZSM-5 catalysts. RSC Advances.
https://doi.org/10.1039/c6ra22492j
Zhang, S. P., Yan, Y. J., Ren, Z., & Li, T. (2003). Study of
hydrodeoxygenation of bio-oil from the fast pyrolysis
of biomass. Energy Sources.
https://doi.org/10.1080/00908310303427
Effect of Variation HCl Concentration on Natural Zeolite Dealumination to the Content of Liquid Smoke Compounds by
Hydrodeoxygenation Process
291
