
Hydrocracking of Mefa Rubber Seed Oil into Biofuels Fraction with
Co and CoMo Metals Supported on Zeolite Catalyst
Junifa Layla Sihombing
1,2
, Herlinawati
2
, Asep Wahyu Nugraha
2
, Ahmad Nasir Pulungan
2
, Moondra
Zubir
2
, Tiamina Nasution
2
, Ary Anggara Wibowo
3
and Saharman Gea
4
1
Postgraduate School, Department of Chemistry, Universitas Sumatera Utara, Jl. Bioteknologi No. 1, Medan, Indonesia
2
Department of Chemistry, Universitas Negeri Medan, Jl. Willem IskandarPasar V Medan Estate, Medan, Indonesia
3
College of Science, Energy Change Institute, College of Science Australian National University, ANU, Australia
4
Department of Chemistry, Universitas Sumatera Utara, Jl. Bioteknologi No. 1, Medan, Indonesia
aryanggarawibowo}@gmail.com,
*
s.gea@usu.ac.id
Keywords: Rubber Seed Oil, Hydrocracking, Catalyst, Impregnation, Biofuel.
Abstract: This research was aimed to produce biofuel fraction from rubber seed oil by using synthetic Zeolite Y (ZY),
Co/ZY and CoMo/ZY catalyst. The insertion of metal used a wet impregnation method. Furthermore, in or-
der to produce a good catalyst, the oxidation and reduction process at 500
o
C using oxygen and nitrogen gas-
es at approximately 2 hours were performed. Then, the effect of addition of ZY, Co/ZY and CoMo/ZY had
been investigated at catalytic hydrocracking using metyl ester fatty acid (MEFA). The reaction was con-
ducted at 450
o
C with ratio of catalyst:feed of 1:4 and 20 mL/sec with hydrogen gas flow. Moreover, the
characterization of catalyst depicted that no significant changes in wavenumber of TO4 groups and a crys-
tallinity of catalyst. Then, the crystal system of ZY and Co/ZY was indicated as monoclinic while Co-
Mo/ZY was revealed as triclinic. The surface area, pore volume and mean pore of catalysts revealed an in-
crease trend as a result of Co and Mo impregnation. These trends have also been proven with SEM analysis
which was shown an equal spreading of metals in the catalysts. Furthermore, the highest result of catalytic
performance among catalysts was shown by CoMo/ZY with 59.45% of bio-gasoline. To sum up, the im-
pregnation of Co and CoMo in Zeolit Y depicted a well-effect on its selectivity into bio-gasoline product.
1 INTRODUCTION
The world's energy needs for petroleum fuels are
increasing in accordance with technological and
industrial developments and the growth of the
world's population. Meanwhile, fossil fuel reserves
continue to decrease and have a negative impact on
the environment. This has encouraged a lot of re-
search to find alternative fuel sources that are sus-
tainable and environmentally friendly. According to
the Renewable Fuel Standard, the consumtion of
renewable fuels currently reaches is 14 billion galos
per year (BGY) and in 2022 is predicted to reach 32
BGY (Perlack et al., 2011).
Indonesia has enormous biomass potential as a
renewable and environmentally friendly alternative
energy sources (Sihombing, Gea, Kembaren, et al.,
2018). Biomass and agricultural or plantation prod-
ucts such as palm oil, soybeans and rubber seed oil
have been converted to biofuels (Vinh et al., 2011).
Rubber seed oil (RSO) is one of the biomass, that is
widely available in Indonesia and has not been wide-
ly used. The main contens of RSO is 39% of linoleic
acid and 23.52% of oleic acid (Wibowo et al., 2014),
which can be converted to liquid fuel fraction by
catalytic cracking process (Sihombing, Gea,
Pulungan, et al., 2018).
Hydrocracking method has been commonly used
to produce biofuels sourced from vegetable oils
(Bezergianni et al., 2009). The use of catalysts in
this process is more desirable because it can reduce
the activation energy (Sriningsih et al., 2014), there-
fore, it will make the process more efficient and can
reduce unnecessary byproducts, such as heteroatom-
ic substances (Khowatimy et al., 2014). In the hy-
drocracking process, breaking the C-C bond from a
long chain carbon compound to a short chain carbon
compound with simultaneous or sequential hydro-
genation. Heterogen catakyst such as Zeolit Y (De
Jong et al., 2010; Pulungan et al., 2014), porous
Layla Sihombing, J., Herlinawati, ., Wahyu Nugraha, A., Nasir Pulungan, A., Zubir, M., Nasution, T., Anggara Wibowo, A. and Gea, S.
Hydrocracking of Mefa Rubber Seed Oil into Biofuels Fraction with Co and CoMo Metals Suppor ted on Zeolite Catalyst.
DOI: 10.5220/0010152200002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 279-286
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
279

material MCM-41 (Zubin et al., 2010) and multi
porous Composite MC-ZSM-5/ MCM-41 [14] have
been used in this process. The fuel products pro-
duced have low density and good physical and
chemical properties as petroleum fuels.
Based on several heterogeneous catalyst types
used, zeolite is the catalyst most widely used in the
petroleum process, because it has a large surface
area, high thermal stability and high acidity
(Degnan, 2000). One type of zeolite which is very
important is Y zeolite. It is widely used as a catalyst
for the process of fluid catalytic cracking, hy-
drocracking, and alkylation in the process of oil
refining and petrochemical synthesis (Choi et al.,
2009). This is because Zeolite-Y has a high concen-
tration of active acid sites, high thermal stability and
high size selectivity (Sato et al., 2001).
One method to increase the activity and selectivi-
ty of the catalyst is by impregnation of transition
metals into zeolites. Co and Mo metals loaded into
pore of alunina have been tested in the heavy oil
hydrocracking and hydrotreating process, which
shows high conversion results and low coke for-
mation (Da Silva, 2014; Parkhomchuk et al., 2013;
Pashigreva et al., 2010). Sihombing, Gea,
Kembaren, et al. (2018) has also been reported that
loaded Co and Mo on natural zeolites as catalysts for
mefa rice bran oil hydrocracking processes have
increased the selectivity of biogasilen products.
Therefore, in this study a modification of the ZY
catalyst character by impregnation of Co and CoMo
metals and an activity test on MEFA hydrocracking
from rubber seed oil to biofuel was carried out. This
modification is expected to provide high conversion
products and good selectivity for the biogasoline
fraction.
2 MATERIALS AND METHODS
2.1 Materials
MEFA Rubber Seeds, Synthetic Zeolite Y (ZY) was
obtained from Tosoh-Japan, Precursor Metal
Co(NO3)2.6H2O, (NH4)6Mo7O24.4H2O were
purchased from Merck. Distilled water was
purchased from CV.Bratachem. Hydrogen Gases,
Oxygen Gases and Nitrogen Gases were purchased
from PT. Aneka Gas.
2.2 Experimental
2.2.1 Preparation of Catalysts
The impregnation of Co and CoMo on ZY according
to Sihombing, Gea, Kembaren, et al. (2018). As
much as 1% (w/w) of Co (NO3)2.6H2O was poured
into 100 grams of zeolite Y through the wet impreg-
nation method. Then, the mixture was refluxed for 5
hours at a temperature of 80
o
C to produce Co-ZY.
After that, the Co-ZY mixture was oxidized with
oxygen gas (± 20 mL/sec) for 2 hours at 500
o
C.
Then, the reduction process was conducted by using
hydrogen gas (±20 mL/sec) for 2 hours at a tempera-
ture of 500
o
C to produce Co/ZY catalyst.
An amount of 1% (w/w) of
(NH4)6Mo7O24.4H2O was poured into 100 grams
of zeolite Y and treated with wet impregnation
method. Then, the mixture was refluxed for 5 hours
at 80
o
C to produce Mo-ZY. After that, a total of 1%
(w/w) of Co(NO3)2.6H2O was added to Mo-ZY. It
was then refluxed for 5 hours at 80
o
C and Co-Mo-
ZY was resulted. The oxygen gas (± 20 mL/sec) was
flowed to Co-Mo-ZY mixture in order to perform
the oxidation process. This was conducted for 2
hours at 500
o
C. Finally, the reduction process was
carried out by using hydrogen gas (± 20 mL/sec) for
2 hours at a temperature of 500
o
C to produce the
CoMo/ZY catalyst.
2.2.2 Characterization of Catalysts
At last, the ZY, Co/ZY and CoMo/ZY catalysts were
analyzed using the FTIR (Shimadzu-Prestige-21),
XRD (shimadzu), SEM (ZEISS Mode EVO MA 10)
and specific surface area measurements with Gas
sorption analyzer through BET method.
2.2.3 Catalyst Activity Test
The activity test of Co/ZY and CoMo/ZY catalysts
towards Catalytic Hydro Cracking process was
performed. MEFA rubber seed oils in a fix-bed
system reactor. The reaction temperature was set at
450 oC and with the ratio of feed:catalyst was 1: 4
with a reaction time of 2 hours [2]. The conversion
yield of each product was calculated using the
equation used by Sriningsih et al. (2014) and the
resulting liquid product was analyzed by GC and
GC-MS method (GC-FID Agilent Technologies
6890N Network).
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
280
3 RESULTS AND DISCUSSIONS
3.1 The Characterization of Catalyst
The catalyst used in this study was synthetic zeolite
Y (ZY). It is a synthetic zeolite which is included in
the classification of faujasite. The cell unit is cube-
shaped with large cell dimensions approaching 25Å
and consists of 192 tetrahedral of (Si, Al)O4. Zeolite
Y is very good for carrying various types of metals
in catalytic hydrocracking reactions since it has a
uniform pore structure and a high concentration of
active sites, so it has excellent thermal stability and
selectivity (Htay & Oo, 2008). In this research, the
ZY catalyst was used by inducing the Co metal and
CoMo metal combination using the wet
impregnation. Then, it was followed by the process
of calcination, oxidation and reduction in the
presence of hydrogen. The calcination process,
oxidation and reduction were carried out to produce
strong interactions between metal and carrier
(Schwarz et al., 1995). From the series of processes,
a uniform distribution of catalyst metal will be
obtained on the surface of the carrier. In addition,
the calcination, oxidation and reduction processes
affect the metal dispersion in the carrier. Metal
preparation of the carrier and the basic properties of
the carrier can affect the physical structure and
chemical properties of metal deposits. The
significant changes to the catalytic properties of a
metal can occur with variations in the composition
of the carrier from different metal development
preparations. To analyze the success of the treatment
given, several analysis of catalyst material were
performed with FTIR, XRD, BET and SEM
methods.
3.1.1 FT-IR Analysis
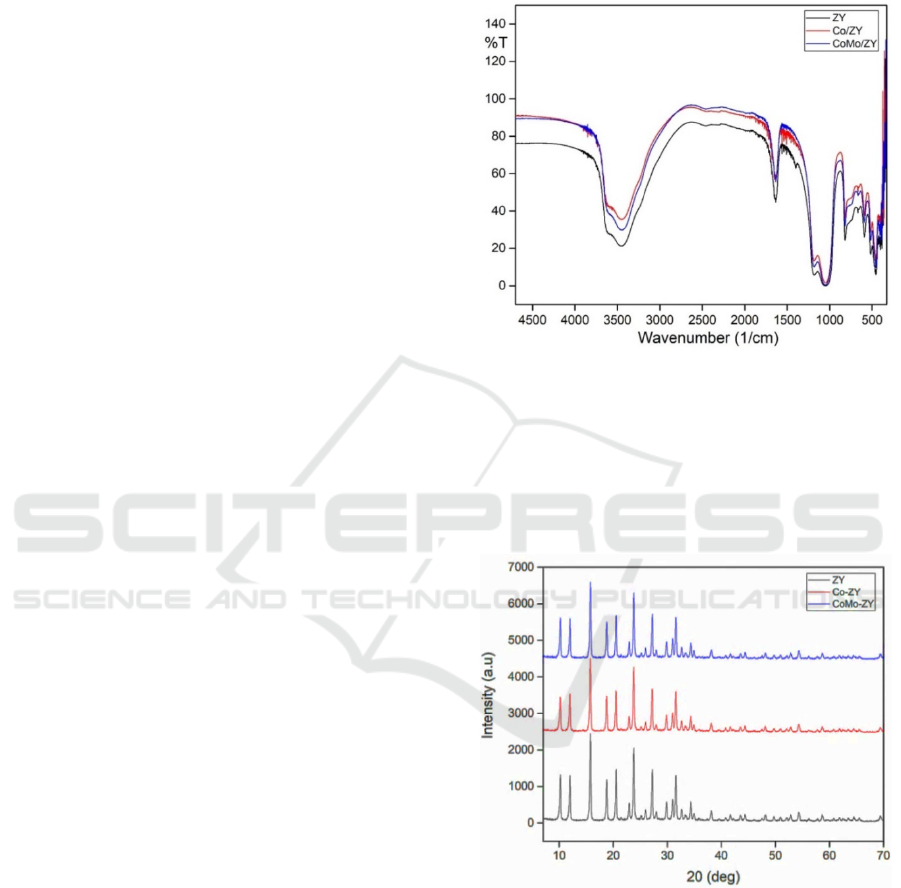
Based on Figure 1, it can be seen that the wave
numbers that show the adsorption of FTIR spectra
on protonated zeolite is at 4000 to 400 cm-1. All
spectra display great intensity at 3451-3442 cm-1
which indicates the vibration of O-H stretching from
Si-OH and Al-OH groups. The spectra recorded at
1642-1635 cm-1 correspond to a buckling vibration
of Si-OH. Then, the peak at 1049-1180 cm-1
indicates the presence of internal and external
asymmetric vibrations of the TiO4 group, while the
peak at 816-817 cm-1 shows the TiO
4
symmetrical
stretching vibration. The intensity of the band
around 591-590 cm-1 indicates the internal buckling
of the tetrahedron while the band around 456-454
cm-1 shows the D4R ring. It can be seen that all
peaks reveal no significant change in the catalyst
after the metal addition. So it can be concluded that
metal development does not contribute damage to
the framework of zeolite.
Figure 1: The comparison of FTIR spectra of ZY, Co/ZY
and CoMo/ZY catalysts.
3.1.2 XRD Analysis
The XRD characterization results are spectra with
abscissa which shows the diffraction and ordinate
angles indicating the intensity.
Figure 2: The XRD diffractogram of ZY, Co/ZY and
CoMo/ZY catalysts.
Based on the comparison of the intensity shown
in Figure 2, it can be inferred that the process of Co
and CoMo metals addition in zeolite Y (ZY) carrier
results in a decrease of some ZY diffraction main
peaks intensity in the range 10-30 θ degrees, yet it
still has the same diffractogram shape. This indicates
that the addition of metal does not damage the crys-
tal structure and the spread of the metal occurs even-
Hydrocracking of Mefa Rubber Seed Oil into Biofuels Fraction with Co and CoMo Metals Supported on Zeolite Catalyst
281
ly on the surface of the ZY pore. The results of this
analysis correlate with the results of the surface area
analysis as shown in Table 2. Further analysis was
carried out using the Expo 2014 method to deter-
mine the catalyst crystal structure. The results of the
analysis are described in Table 1.
Table 1 reveals that the addition of Co metal
does not change the ZY carrier crystal system. How-
ever, the crystal system change was occurred in
CoMo / ZY. This is probably the result of strong
interaction of Co and Mo metals on the pore surface.
The Interactions between metals can cause the for-
mation of alloys. Moreover, alloy formation in the
ZY carrier pore will cause a geometric change due to
the limitation of the size of each component.
Table 1: The result of XRD data analysis on ZY, Co/ZY and CoMo/ZY catalysts via EXPO method.
Catalysts A (Å) B (Å) C (Å) a (
0
) b (
0
) g (
0
) Vol (Å
3
)
Crystal
System
ZY 8.14904 8.98075 12.48393 117.9507 122.3303 91.5312 633.1 Triclinic
Co/ZY 10.26336 11.39037 10.24643 60.73391 67.99091 48.9241 785.11 Triclinic
CoMo/ZY 14.33625 8.65771 7.50814 89.99995 100.0584 89.99995 917.58 Monoclinic
3.1.3 BET Method Analysis
Table 2 shows the results of determining the catalyst
surface area using the Bruener-Emmer-Teller (BET)
method. From these data, it can be seen that the
addition of Co and CoMo metals on ZY increases
the total pore volume of ZY catalyst. This indicates
that the metals were spread evenly on the pore sur-
face of the ZY carrier. In contrast to the results of
the measurement of the pore spacing, the addition of
metal into the pore of the ZY carrier results in a
greater average pore radius. This is possible since
the more metals are impregnated into the pores of
the carrier, and then the pores of the carrier with
smaller fingers will be more clogged so that the
average pore radius increases. Meanwhile, the cata-
lyst surface area after metal Co has increased from
342.651 m2g-1 (ZY) to 491.246 m2g-1 (Co / ZY),
but it has decreased with CoMo/ZY metal addition
with the value of 435.239 m2g-1. However, when it
is compared to basic ZY catalysts, the addition of Co
and CoMo increases the catalyst specific surface
area. Similar results were reported by Semeykina et
al. (2016), showing that Co, Mo and Ni metals in
mesoporous alumina (Al2O3) carriers increased the
catalyst specific surface area. Surface area, pore
volume and mean pore size are important character-
istics of the catalyst which greatly affect the activity
and selectivity of the catalyst. The scheme of metal
loaded to the ZY catalyst presented in figure 3.
Table 2: The result of BET analysis on ZY, Co/ZY a
CoMo/ZY catalysts.
Catalyts Area
(m
2
g
-1
)
Pores
Volume
(
cc/
g
r
)
Average
Pore Size
(
Å
)
ZY 342.651 0.24 13.78
Co/ZY 491.246 0.40 16.38
CoMo/ZY 435.239 0.66 30.43
3.1.4 SEM Analysis
SEM (Scanning Electron Microscopic) analysis is
used to determine the catalyst surface topology.
Moreover, it aims to determine the dispersion level
of the metal which is applied to ZY. The visualiza-
tion of the Co and CoMo addition on the ZY carrier
pliers was evenly distributed on the surface of the
ZY carrier pore illustrated in Figure 4. The result of
SEM analysis is shown in Figure 4, which shows
that the distribution of metals in ZY is evenly dis-
tributed and does not show sintering metals on the
pore surface of the ZY carrier. This is indicated by
the shape of the surface morphology of each catalyst
which is relatively homogeneous. This SEM data
correlates with previous XRD and BET data.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
282
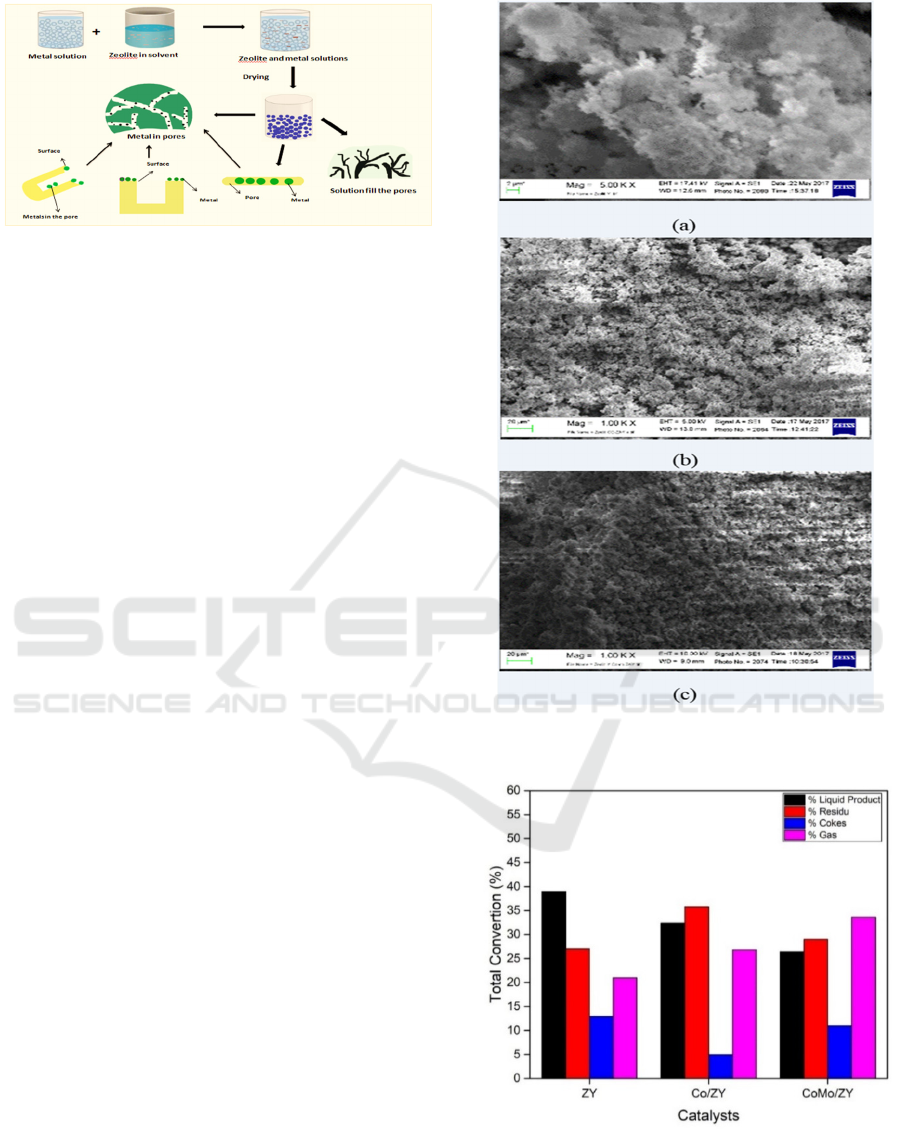
Figure 3: The scheme of metal loaded to the ZY catalyst.
3.2 Catalytic Performance of Catalyst
The catalyst activity test was carried out on the hy-
drocracking reaction of rubber seed oil. The feed
solution used was MEFA from rubber seed oil with
the ratio of catalyst and feed used was 1:4 (w/w).
Rubber seed oil MEFA hydrocracking reaction was
carried out at a temperature of 450
o
C with hydrogen
gas flow of 20 mL/minute for 2 hours. The products
which were produced are gas, liquid and coke. The
resulting liquid product was analyzed by GC and
GC-MS. The coke formation can be seen from
changes in the color of the catalyst which tends to be
darker after the reaction process takes place. The
results of the activity and selectivity test of each
catalyst are presented in Figure 5 and figure 6. Fig-
ure 5 show that the metal addition of the Co/ZY
catalyst gives a larger gas product than the ZY cata-
lyst which was 26.60%. This is caused by the entry
of Co metal in the carrier pore gives more active
sites which play a role in the reaction process. Zhang
et al., 2003 reported that homogeneously distributed
metals on zeolite surfaces and pores had increased
catalytic active sites. In addition, the Co/ZY catalyst
has a larger surface area, so the probability of the
reaction occurring in the active site is greater. How-
ever, in CoMo/ZY Catalyst, it provides a larger gas
product than Co/ZY catalyst. This was considered
from the role of the combination of Mo metal as a
catalyst promoter which showed a performance in
increasing catalyst activity in the MEFA hy-
drocracking reaction of rubber seed oil. The conver-
sion of liquid products produced from each ZY,
Co/ZY and CoMo/ZY catalysts were 39%, 32.4%
and 26.4% respectively. The selectivity of liquid
products to the gasoline and diesel fractions is dis-
played in Figure 6.
Figure 4: The SEM analysis result of (a) ZY, (b) Co/ZY
and (c) CoMo/ZY catalysts.
Figure 5: The catalytic activity of MEFA hydrocracking of
rubber seed oil with ZY, Co/ZY and CoMo/ZY catalyst at
a temperature of 450 oC with the ratio of 1:4.
Hydrocracking of Mefa Rubber Seed Oil into Biofuels Fraction with Co and CoMo Metals Supported on Zeolite Catalyst
283
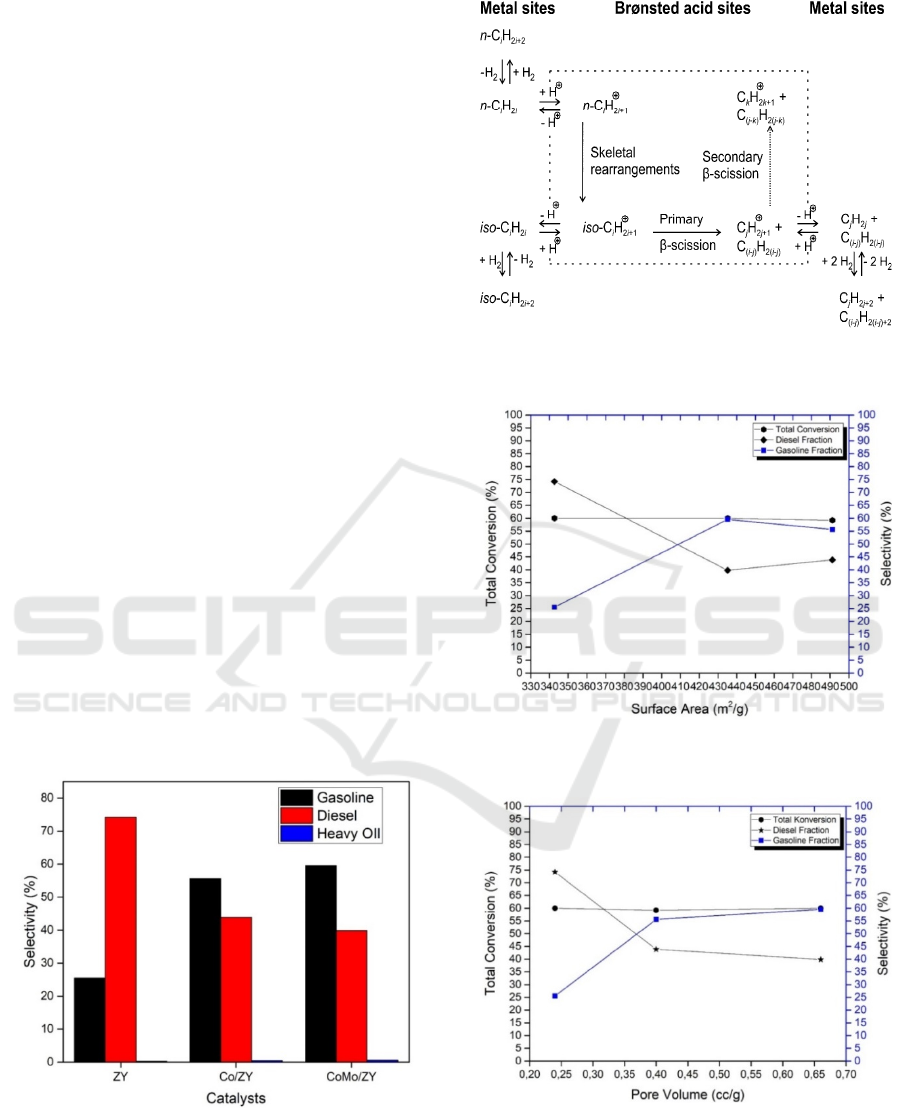
Figure 6 shows that the ZY catalyst produced by
liquid hydrocarbons is dominated by diesel fractions
with 74.18%. However, for both the Co/ZY and
CoMo/ZY catalysts, the selectivity of liquid hydro-
carbon products produced on the gasoline fraction
increased significantly with the percentage of selec-
tivity to gasoline with 55.58% and 59.45% respec-
tively. The metal catalyst system of Co/ZY and
CoMo/ZY is able to increase the product selectivity
to the gasoline fraction to be more than 2 times that
of using a metal-free catalyst (ZY). This is due to the
increase in catalyst surface area and pore volume.
Trisunaryanti et al. (2013) reported that by using
zeolites which is containing metal, it will produce
liquid products with the C7-C12 fuel fractions. This
phenomenon may occur due to the presence of the
Bronsted acid site and as an active metal site causing
the solids to have catalytic properties. The empty d
orbitals owned by the metal, is functioning as Lewis
acid sites which can accept electron pairs from the
reactants. Therefore, the breakdown of the C-C
bonds occurs through the carbocation mechanism.
The CoMo/ZY catalyst shows the role of a combina-
tion of Co and Mo metals, where Mo acts as a pro-
moter on the catalyst which contributes to the in-
crease in catalyst activity in the MEFA rubber seed
oil hydrocracking reaction. This causes the Co-
Mo/ZY catalyst to produce gas and gasoline prod-
ucts that are larger than the ZY and Co/ZY catalysts.
The mechanism of the hydrocracking reaction on the
metal-carrying system catalyst (bifunctional cata-
lyst) has been described by Weitkamp (2012), as
described in Figure 7.
Figure 6: The selectivity of liquid product of MEFA hy-
drocracking of rubber seed oil with ZY, Co/ZY and Co-
Mo/ZY catalyst at a temperature of 450
o
C with the ratio of
1:4.
Figure 7: The classical reaction mechanism of n-alkane
hydrocracking process on metal addition catalytic system.
Figure 8: The relation between surface area of catalyst to
the total conversion and selectivity level.
Figure 9: The relation between pore volume of catalyst to
the total conversion and selectivity level.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
284
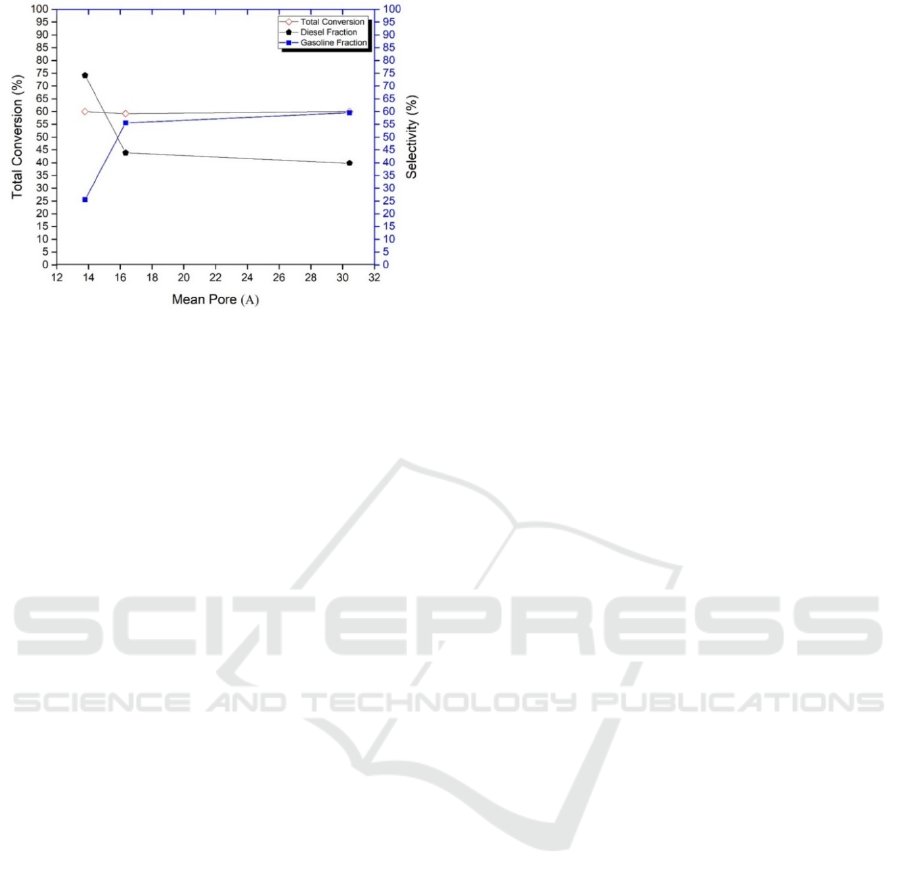
Figure 10: The relation between mean pore of catalyst to
the total conversion and selectivity level.
The relationship between specific surface area,
pore volume and mean of pore catalyst to the total
conversion and product selectivity are shown in
Figure 8, 9 and 10. Figure 8 shows that the increase
in catalyst surface area, will not giving a significant
effect on the total conversion results. In the other
hand, increasing the catalyst surface shows a ten-
dency to produce a higher gasoline fraction. This
condition is inversely proportional to the selectivity
of the diesel fraction. This is possible since the wide
catalyst surface provides a higher chance of adsorp-
tion and cracking reactions to produce lighter frac-
tions of gasoline or hydrocarbons. The same trends
are also shown in Figure 9 and 10. In Figure 9 and
10, it can be observed that the increase in pore vol-
ume and the mean of the catalyst pore range does
not contribute large influence on the total conversion
value, but shows the trend of increasing the selec-
tivity of liquid products to the gasoline fraction.
Increased pore volume as a result of the widening of
the pore size of the catalyst, so that the diffusion
process of the molecules resulting from cracking
MEFA can penetrate the pore, no further reaction
occurs, resulting in hydrocarbon compounds which
is equal to gasoline fractions in higher amounts. This
phenomenon may also be caused by the suitability of
the pore size of the catalyst at the time of cracking
reaction, including the process of reactant adsorp-
tion, catalytic cracking process, and desorption of
cracking molecules from the catalyst surface.
4 CONCLUSIONS
The Co and CoMo metal supported on ZY carriers
exhibit characteristics in increasing catalyst specific
surface area, total pore volume and mean catalyst
pore rate. The data obtained illustrate that Co metal
and CoMo metal presences do not damage the crys-
tal structure of ZY and are evenly distributed on the
pore surface of the carrier. From the processing of
XRD data using expo-2014 method, it was found
that ZY and Co/ZY catalysts were formed with a
triclinic crystal system, while for CoMo/ZY cata-
lysts showed a monoclinic crystal system. Moreover,
in the rubber seed oil MEFA hydrocracking reaction,
ZY catalyst showed a 74.18% higher selectivity for
biodiesel products. The presence of Co and CoMo
metals loaded on ZY increases the activity and se-
lectivity to the formation of the gasoline fraction. To
sum up, the highest gasoline product was produced
by CoMo/ZY catalyst with 59.45% w/t, followed by
Co/ZY catalyst with 55.58% w/t.
ACKNOWLEDGEMENT
Authors would like to acknowledge Rector Universi-
tas Negeri Medan and LPPM-Unimed for financial
support via “Hibah Penelitian KDBK-2019” scheme.
Moreover, a grateful acknowledgement is addressed
to Universitas Negeri Medan Research Center, Indo-
nesia, for facilitating the research.
REFERENCES
Bezergianni, S., Kalogianni, A., & Vasalos, I. A. (2009).
Hydrocracking of vacuum gas oil-vegetable oil mix-
tures for biofuels production. Bioresource Technology,
100(12), 3036–3042.
Choi, M., Na, K., Kim, J., Sakamoto, Y., Terasaki, O., &
Ryoo, R. (2009). Stable single-unit-cell nanosheets of
zeolite MFI as active and long-lived catalysts. Nature,
471(7261).
Da Silva, A. C. V. (2014). Study of the liquid activation of
CoMo and NiMo cat-alysts.
De Jong, K. P., Zečević, J., Friedrich, H., de Jongh, P. E.,
Bulut, M., Van Donk, S., & Fajula, F. (2010). Zeolite
Y crystals with trimodal porosity as ideal hy-
drocracking catalysts. Angewandte Chemie
International Edition, 49(52), 10074–10078.
Degnan, T. F. (2000). Applications of zeolites in
petroleum refining. Topics in Catalysis, 13(4), 349–
356.
Htay, M. M., & Oo, M. M. (2008). Preparation of Zeolite
Y catalyst for petroleum crack-ing. World Academy of
Science, Engineering and Tech-Nology, 48, 114–120.
Khowatimy, F. A., Priastomo, Y., Febriyanti, E.,
Riyantoko, H., & Trisunaryanti, W. (2014). Study of
waste lubricant hydrocracking into fuel frac-tion over
the combination of Y-zeolite and ZnO cata-lyst.
Procedia Environmental Sciences, 20, 225–234.
Hydrocracking of Mefa Rubber Seed Oil into Biofuels Fraction with Co and CoMo Metals Supported on Zeolite Catalyst
285

Parkhomchuk, E. V., Lysikov, A. I., Okunev, A. G.,
Parunin, P. D., Semeikina, V. S., Ayupov, A. B., &
Parmon, V. N. (2013). Meso/macroporous CoMo
alumina pellets for hy-drotreating of heavy oil.
Industrial & Engineering Chemistry Research, 52(48),
17117–17125.
Pashigreva, A. V., Bukhtiyarova, G. A., Klimov, O. V.,
Chesalov, Y. A., Litvak, G. S., & Noskov, A. S.
(2010). Activity and sulfidation behavior of the
CoMo/Al2O3 hydrotreating catalyst: The effect of
drying conditions. Catalysis Today, 149(1–2), 19–27.
Perlack, R. D., Eaton, L. M., Turhollow Jr, A. F., Lang-
holtz, M. H., Brandt, C. C., Downing, M. E., &
Nelson, R. G. (2011). US billion-ton update: biomass
supply for a bioenergy and bioproducts industry.
Pulungan, A. N., Sihombing, J. L., Nasution, H. I., Evina,
R., Dibyantini, Selly, R., Trisunaryanti, W., &
Triyono. (2014). Preparation, Characterization and
Activity Assay of NiO-CoOMoO/Zeolite-Y Catalyst
on Hydrocracking of Casher Nut Shell Liquid in
Fixed-bed Reactor. The First International Seminar on
Trends in Science and Science Education.
Sato, K., Nishimura, Y., Honna, K., Matsubayashi, N., &
Shimada, H. (2001). Role of HY zeolite mesopores in
hydrocracking of heavy oils. Journal of Catalysis,
200(2), 288–297.
Schwarz, J. A., Contescu, C., & Contescu, A. (1995).
Methods for preparation of catalytic materials.
Chemical Reviews, 95(3), 477–510.
Sihombing, J. L., Gea, S., Kembaren, A., Pulungan, A. N.,
Wibowo, A. A., & Wirjosentono, B. (2018). Activity
assays of calcinated sarulla natural zeolite (snz-cal) in
catalytic hydrocracking rubber seed oil. Journal of
Physics: Conference Series, 1116(4).
Sihombing, J. L., Gea, S., Pulungan, A. N., Agusnar, H.,
Wirjosentono, B., & Hutapea, Y. A. (2018). The
characterization of Sarulla natural zeolite crystal and
its morphological structure. AIP Conference
Proceeding, 2049(1).
Sriningsih, W., Saerodji, M. G., Trisunaryanti, W.,
Armunanto, R., & Falah, I. I. (2014). Fuel production
from LDPE plastic waste over natural zeolite
supported Ni, Ni-Mo, Co and Co-Mo metals. Procedia
Environmental Sciences, 20, 215–224.
Trisunaryanti, W., Syoufian, A., & Purwono, S. (2013).
Characterization and modification of indonesian natu-
ral zeolite for hydrocracking of waste lubricant oil into
liquid fuel fraction. Journal of Chemistry and
Chemical Engineering, 7(2), 175.
Vinh, T. Q., Loan, N. T. T., Yang, X. Y., & Su, B. L.
(2011). Preparation of bio-fuels by catalytic cracking
reaction of vegetable oil sludge. Fuel, 90(3), 1069–
1075.
Weitkamp, J. (2012). Catalytic hydrocracking—
mechanisms and versatility of the process.
ChemCatChem, 4(3), 292–306.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
286
