
Morphological Investigation of Electrospun Nanofibers Cellulose
Acetate-based Membrane
Aditia Warman
1
, Hamonangan Nainggolan
2
, Mahyuni Harahap
2
, Dellyansyah
3
, Grace Nainggolan
3
,
Suhut Alexander Situmorang
2
, Saharman Gea
2*
1
Department of Physics, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Jalan Bioteknologi
Padang Bulan, Medan, 20155, Sumatera Utara,Indonesia
2
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Jalan Bioteknologi
Padang Bulan, Medan, 20155, Sumatera Utara,Indonesia
3
Postgraduate Chemistry Study Program, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jalan Bioteknologi Padang Bulan, Medan, 20155, Sumatera Utara,Indonesia
Keywords: Nanofibers, Electrospinning, Cellulose Acetate, Membrane, Morphology
Abstract: Electrospinning technique had been used to produce ultra-fine fiber with diameter in nano-scale by dissolving
polymer precursor in suitable solvents. In this study, electrospinning was used to produce cellulose acetate
nanofibers with distance 20 cm, flowrate 0.10-0.15 ml/h and voltage 8 kV. Cellulose acetate was dissolved in
acetone:DMSO (2:8 and 3:7). The morphologies of the spun fibers were investigated using a scanning electron
microscope (SEM). The functional group of the fibers was analyzed using a Fourier Transform Infrared
(FTIR). The result of FTIR showed that C=O functional group at wavenumber 1751 cm
-1
. The morphology
of cellulose acetate dissolved in acetone:DMSO (3:7) wassmoother than acetone:DMSO(2:8).
1 INTRODUCTION
About the population of the world, 11% of themis
lack clean water access. The World health
Organization (WHO) in 2012 anticipates that the
shortage of clean water can involve with 4 million
lives by 2050. Although water consists of more than
70% on earth surface, 97% is sea water (Shirazi,
Kargari and Shirazi, 2012). In addition, almost 3% of
the water stuck in the ground or inside glaciers and
ice. Hence, the world only leaves less than 1% of
water that can be depleted, the desire of gaining more
productive and economical water filtration and
cleaning methods are required to raise demand for
water.
Membrane-based technology has gained
popularity for more than a last century due to its high
separation efficiency, inexpensive and easy to
operate. The membrane work on principle two phases
separation which only let the phase with suitable size
of membrane porous. Membrane can be classified
into porous and dense depending of their structure
(Takht Ravanchi, Kaghazchi and Kargari, 2009). The
nature of membrane transport and selectivity depends
heavily on the structure of its porous (Ahmed, Lalia
and Hashaikeh, 2015).
Nanofibers are part of a nanomaterial which has
very unique and interesting properties due its
nanoscale diameters and large aspect ratio(Huang et
al., 2003). They can be produced by various
technique such as synthesis templates, phase
separation(Ichimori et al., 2013), self-assembly,
steam-explosion(Gea et al., 2018), and
electrospinning(Arkoun et al., 2017). Among three of
them, electrospinning develops significantly due its
simple and reliable technique for converting various
polymer into with controllable morphology(Ahmed,
Lalia and Hashaikeh, 2015) .Electrospinning is a
technique used to produce a continuous of nanofibers
in non-woven form. The process spinning fibers
diameters ranging from 80 to several hundred
nanometers. The report on electrospinning
continuous to increase due to its efficient equipment
and usage, simple, fast and economical (Daels et al.,
2011).
One of nanofiber applications is water filtration.
For flat-shaped nanofiber, it can be applied as a water
filtration membrane or microfiltration. Due to the
higher porosity and interconnected open pore
Warman, A., Nainggolan, H., Harahap, M., Dellyansyah, ., Nainggolan, G., Alexander Situmorang, S. and Gea, S.
Morphological Investigation of Electrospun Nanofibers Cellulose Acetate-based Membrane.
DOI: 10.5220/0010152000002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 267-272
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
267
structure provide higher permeability properties for
filtration of water than conventional materials
(Bjorge et al., 2009). The purpose of this study is to
produce electrospun nanofiber membranes of
cellulose acetate and investigate its morphology.
Cellulose acetate is an easily dissolved polymer in
acetone, but the acetone is highly volatile with a
boiling point of about 56ºC. In electrospinning the use
of volatile solvent causes the fibers to evaporate
before reaching the collector, it would block the tip of
the needle and stop the spinning process(Son et al.,
2004). Thus, acetone needs to be lowered in its
volatility by adding other solvents to form binary
solvent including acetone-water (Son et al., 2004;
Quirós et al., 2016), acetone-ethanol (Baptista et al.,
2011), and acetone-DMAc (Tungprapa et al., 2007).
The use of water, ethanol, acetic acid, and DMAc
mixed with acetone has many disadvantages such as
the ratio of water should be smaller, the volatility of
ethanol approaching acetone, and the corrosive
DMAc and acetic acid harm the electrospinning
device. In this study, we were interested in using
acetone and DMSO as binary solvent to dissolve
cellulose acetate. Then cellulose acetate solution was
fabricated into nanofiber by using electrospinning.
2 MATERIALS AND METHODS
2.1 Materials and Devices
Table 1 : Summary of chemical materials used in this
study
Properties
Cellulose
acetate
Acetone DMSO
Form
Density (g/ml) at
25ºC
Solid
1.3
Liquid
0.791
Liquid
1.1
Boiling
Temperature ºC
no data 56 189
Molecular weight
(g/mol)
Mn 30000 by
GPC
58.08 78.13
Chemical materials included cellulose acetate
(CA), acetone and dimethyl sulfoxide (DMSO) were
obtained from Sigma Aldrich.Devices usedinclude
terumo 10 cc srynge and electrospinning series device
from Nanolab instrument (NLi) Company Malaysia.
The summary of chemical is written on Table 1.
2.2 Preparation of Electrospun
Nanofibers
Cellulose acetate (CA) solution was prepared with
concentration 17.5% and 20% (w/v) by dissolvingCA
in acetone:DMSO 2:8 and 3:7 (v:v)under reflux at 40-
50
o
C for 2 hours.TheCA solution wasthen transferred
into10 cc terumo srynge. The electrospinning was run
with flowrate 0.10-0.15 ml/h, distance 20 cm and
voltage 8 kV at room temperature.The spun-
nanofibers were collected using a flat collector.
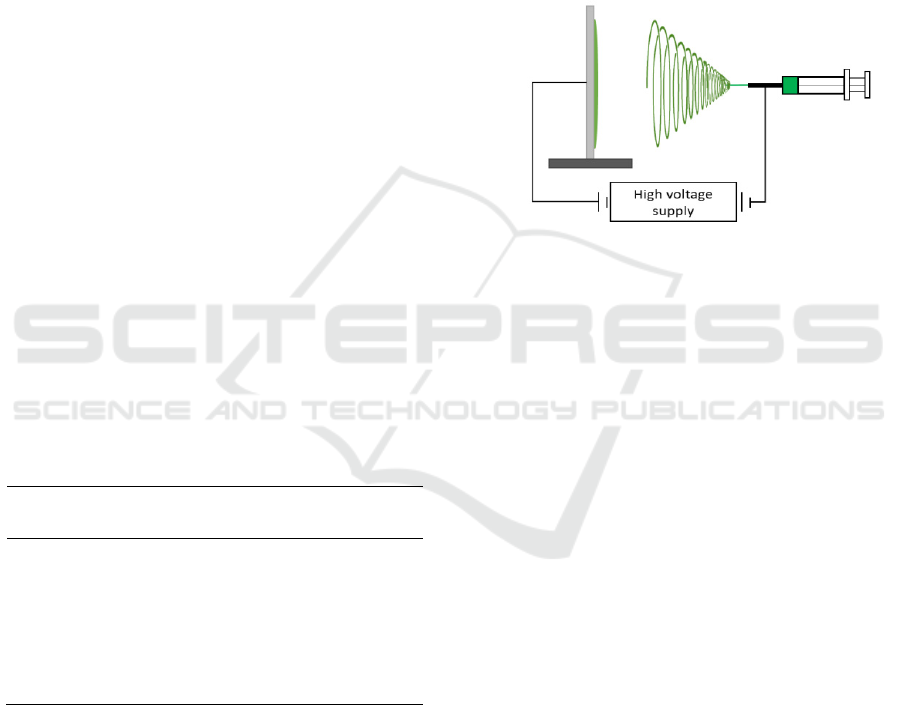
Electrospinning set up is illustrated in
Figure 1.
Figure 1: Electrospinning set up.
2.3 Characterization
2.3.1 Scanning Electron Microscope
The morphology of the fibers was analyzed using a
scanning electron microscope Jeol 6060. To reduce
charging during analysis, the fibers were sputter-
coated with a layer of gold alloy.
2.3.2 Fourier Transform Infrared
The functional group of raw cellulose acetate and
spun-fibers was analyzed using an FTIR
Spectrometer (Nicolet 8700, Thermo Scientific). The
instrument was operated in a transmission-mode with
a resolution of 2 cm
-1
and 100 scans.
3 RESULTS AND DISCUSSION
3.1 Morphology Analysis
The morphology of electrospun nanofibers was
investigated using SEM. Figure 2 and Figure 3 show
the micrographs of CA nanofibers in
acetone:DMSO(2:8 and 3:7). The average diameter of
the spun fibres is written in
Table 2.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
268

Figure 2: The SEM image of spun fiber: (a)-(b) CA 17.5 % and (c)-(d) CA 20 % (w/v) in acetone:DMSO 2:8 (v:v)
Figure 3: The SEM image of spun fiber: (a)-(b) CA 17.5 % and (c)-(d) CA 20 % (w/v) in acetone:DMSO 3:7 (v:v)
Figure 2 shows that the morphology at CA 20%
was smoother than CA 17.5 %. However, it had bead
fibers. The bead fiber is probably related to the
electrospun jet which get capillary breakup by surface
tension (Fong, Chun and Reneker, 1999).
Additionally, Figure 3 shows that both the
morphology at CA 17,5% had smooth and narrow
morphology almost the same as the morphology of
CA20% cellulose acetate. However, there were some
particles left on the surface of the fiber.
The average diameters data as written in Table 2
explains
that the fiber with CA 20 % had larger
Morphological Investigation of Electrospun Nanofibers Cellulose Acetate-based Membrane
269
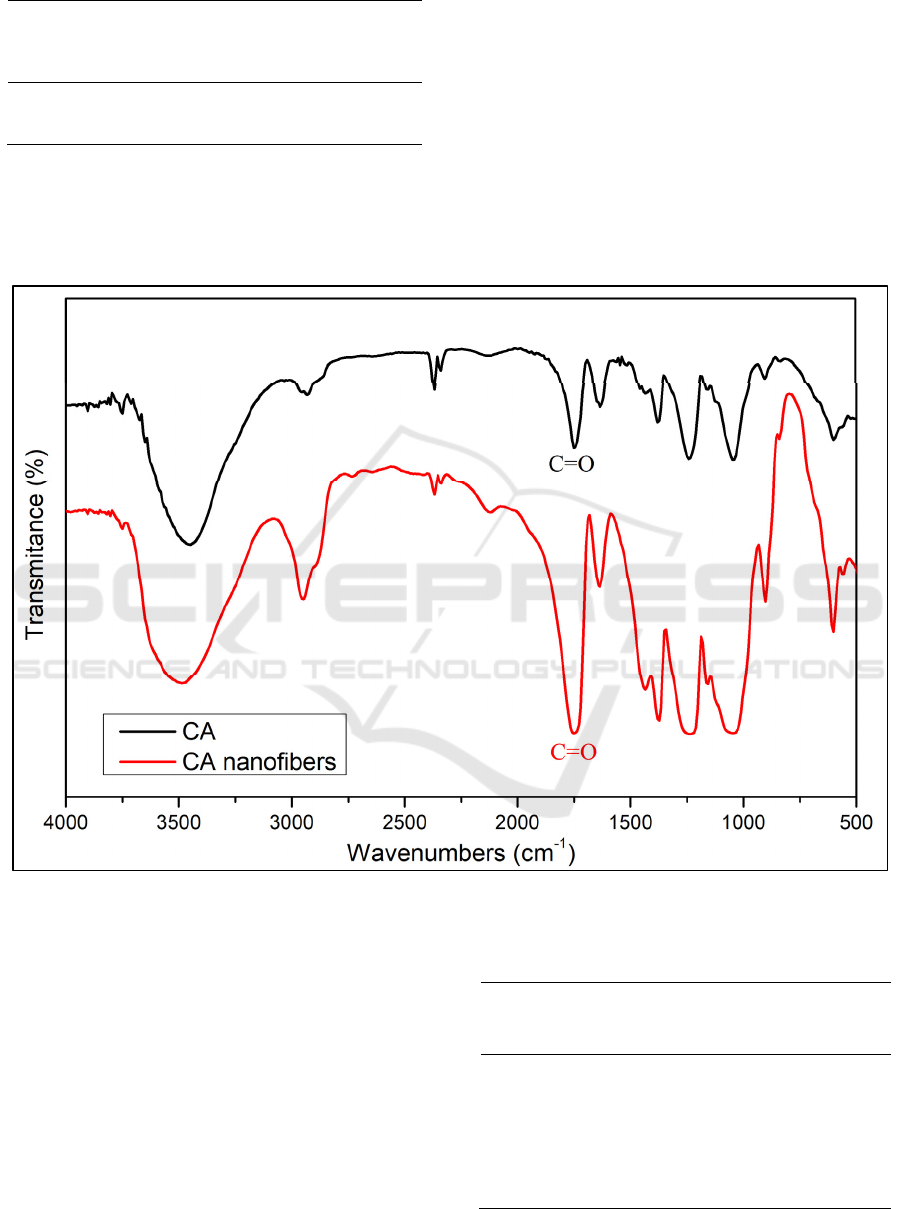
Table 2: The average diameter of CA nanofibers.
ratio of
acetone :
DMSO (v/v)
fibers diameters (nm)
17.5 % cellulose
acetate
20 % cellulose
acetate
2:8 301.7 152.2
3:7 165.8 212.1
diameter than CA 17.5 % in acetone:DMSO (3:7).
Higher polymer concentration causes increasing of
fiber diameters as reported in reference (Beachley and
Wen, 2009).In addition, both of fibers in had larger
diameters than the fiber at CA 20 % in
acetone:DMSO (2:8).The larger ratio of volatile
solution also increases diameter of fiber (Tungprapa
et al., 2007). However, the fiber at CA 17.5 % in
acetone DMSO (2:8) had unique attention, whereas
its diameter was larger among all fibers.
3.2 Fourier Transfer Infra-red
Analysis
The FTIR spectra of CA nanofibers and cellulose
acetate powder areshown in Figure 4, and the
wavenumbers of each functional groupare written in
Table 3.
Figure 4 : FTIR spectra of CA and CA nanofibers
The FTIR spectra of CA nanofibers showed broad
peak of O-H stretching at 3487 cm
-1
and medium peak
of CH; CH
2
or CH
3
stretching at 2947 cm
-1
(John,
Chen and Kim, 2012). Strong peak which attributes
to C=O functional groupat 1435 cm
-1
and C-O group
at peak 1049 cm
-1
(Ibrahim et al., 2015).
Table 3. The FTIR data of CA and CA nanofiber used in
this study.
Group
Wavenumbers (cm
-1
)
Cellulose
acetate
Cellulose acetate
nanofibe
r
O-H 3449 3487
C-H 2932 2947
C=O 1751 1751
C=C 1636 1636
CH
2
1434 1435
C-O 1042 1049
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
270

The C=O group became stronger and slightly
wider after fabrication of cellulose acetate into
nanofiber. It was due to the presence of C=O from
acetone which appeared at 1770-1730 cm
-1
(Hasan,
Zaki and Pasupulety, 2003). The peak at 1049 cm
-1
of
nanofiber was wider than cellulose acetate. From a
reference reported that the peak S=O of DMSO
appears at 1070 to 1030 cm
-1
(Awadhia and Agrawal,
2007). In this study, DMSO was used in high ratio.
Hence the presence of DMSO affected the peak of IR
spectrum.
4 CONCLUSION
The fabrication of nanofiber from cellulose acetate
dissolved in acetone:DMSO has been done using
electrospinning. The morphology of the nanofiber
dissolved in acetone:DMSO (3:7) is smoother than
acetone:DMSO (2:8). The peak intensity of C=O at
1751 cm
-1
becomes stronger after fabrication of
nanofiber.
ACKNOWLEDGEMENT
The authors acknowledge to Rector of Universitas
Sumatera Utara 2019 for financial support through
TALENTA 2019 with contract number :
4167/UN5.1.R/PPM/2019.
REFERENCES
Ahmed, F. E., Lalia, B. S. and Hashaikeh, R. (2015) ‘A
review on electrospinning for membrane fabrication:
Challenges and applications’, Desalination. Elsevier
B.V., 356, pp. 15–30. doi: 10.1016/j.desal.2014.09.033.
Arkoun, M. et al. (2017) ‘Antibacterial electrospun
chitosan-based nanofibers: A bacterial membrane
perforator’, Food Science and Nutrition, 5(4), pp. 865–
874. doi: 10.1002/fsn3.468.
Awadhia, A. and Agrawal, S. L. (2007) ‘Structural, thermal
and electrical characterizations of
PVA:DMSO:NH4SCN gel electrolytes’, Solid State
Ionics, 178(13–14), pp. 951–958. doi:
10.1016/j.ssi.2007.04.001.
Baptista, A. C. et al. (2011) ‘Thin and flexible bio-batteries
made of electrospun cellulose-based membranes’,
Biosensors and Bioelectronics. Elsevier B.V., 26(5),
pp. 2742–2745. doi: 10.1016/j.bios.2010.09.055.
Beachley, V. and Wen, X. (2009) ‘Effect of electrospinning
parameters on the nanofiber diameter and length’,
Materials Science and Engineering C. Elsevier B.V.,
29(3), pp. 663–668. doi: 10.1016/j.msec.2008.10.037.
Bjorge, D. et al. (2009) ‘Performance assessment of
electrospun nanofibers for filter applications’,
Desalination, 249(3), pp. 942–948. doi:
10.1016/j.desal.2009.06.064.
Daels, N. et al. (2011) ‘Potential of a functionalised
nanofibre microfiltration membrane as an antibacterial
water filter’, Desalination. Elsevier B.V., 275(1–3), pp.
285–290. doi: 10.1016/j.desal.2011.03.012.
Fong, H., Chun, I. and Reneker, D. H. (1999) ‘Beaded
nanofibers formed during electrospinning’, Polymer,
40(16), pp. 4585–4592. doi: 10.1016/S0032-
3861(99)00068-3.
Gea, S. et al. (2018) ‘The Isolation of Nanofibre Cellulose
from Oil Palm Empty Fruit Bunch Via Steam Explosion
and Hydrolysis with HCl 10%’, Journal of Physics:
Conference Series, 979(1), pp. 0–8. doi: 10.1088/1742-
6596/979/1/012063.
Hasan, M. A., Zaki, M. I. and Pasupulety, L. (2003) ‘Oxide-
catalyzed conversion of acetic acid into acetone: An
FTIR spectroscopic investigation’, Applied Catalysis
A: General, 243(1), pp. 81–92. doi: 10.1016/S0926-
860X(02)00539-2.
Huang, Z. M. et al. (2003) ‘A review on polymer nanofibers
by electrospinning and their applications in
nanocomposites’, Composites Science and Technology,
63(15), pp. 2223–2253. doi: 10.1016/S0266-
3538(03)00178-7.
Ibrahim, M. M. et al. (2015) ‘Role of tosyl cellulose acetate
as potential carrier for controlled drug release’, Life
Science Journal, 12(10), pp. 127–133. doi:
10.7537/marslsj121015.16.
Ichimori, T. et al. (2013) ‘Morphological diversity and
nanofiber networks of poly(p-oxybenzoyl) generated
by phase separation during copolymerization’, Journal
of Applied Polymer Science
, 128(2), pp. 1282–1290.
doi: 10.1002/app.38554.
John, A., Chen, Y. and Kim, J. (2012) ‘Synthesis and
characterization of cellulose acetate-calcium carbonate
hybrid nanocomposite’, Composites Part B:
Engineering. Elsevier Ltd, 43(2), pp. 522–525. doi:
10.1016/j.compositesb.2011.08.021.
Quirós, J. et al. (2016) ‘Electrospun cellulose acetate
composites containing supported metal nanoparticles
for antifungal membranes’, Science of the Total
Environment. Elsevier B.V., 563–564, pp. 912–920.
doi: 10.1016/j.scitotenv.2015.10.072.
Shirazi, M. M. A., Kargari, A. and Shirazi, M. J. A. (2012)
‘Direct contact membrane distillation for seawater
desalination’, Desalination and Water Treatment,
49(1–3), pp. 368–375. doi:
10.1080/19443994.2012.719466.
Son, W. K. et al. (2004) ‘Preparation of antimicrobial
ultrafine cellulose acetate fibers with silver
nanoparticles’, Macromolecular Rapid
Communications, 25(18), pp. 1632–1637. doi:
10.1002/marc.200400323.
Takht Ravanchi, M., Kaghazchi, T. and Kargari, A. (2009)
‘Application of membrane separation processes in
petrochemical industry: a review’, Desalination.
Morphological Investigation of Electrospun Nanofibers Cellulose Acetate-based Membrane
271

Elsevier B.V., 235(1–3), pp. 199–244. doi:
10.1016/j.desal.2007.10.042.
Tungprapa, S. et al. (2007) ‘Electrospun cellulose acetate
fibers: Effect of solvent system on morphology and
fiber diameter’, Cellulose, 14(6), pp. 563–575. doi:
10.1007/s10570-007-9113-4.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
272
