
Utilization of Coconut Waste as a Basic Material for Making Carbon
Dots with Acid Oxidation Method
Marpongahtun
1,2
,
Irmayani
1
and Suci A.
1
1
Chemistry Department, Universitas Sumatera Utara, Jalan Bioteknologi No. 1, Medan, Indonesia
2
Laboratorium Penelitian Terpadu Universitas Sumatera Utara, Medan, Indonesia
Keywords: Carbon Dots, Fluorescence, Soot, Acid Oxidation.
Abstract: Utilization of coconut shell soot as raw material for making carbon dots has been successfully carried out.
This study used coconut shell soot with 5 M HNO
3
acid oxidation method which is then the Carbon dots
characteristics were determined using UV light, UV-Vis, TEM analysis, and functional group analysis with
FT-IR. Coconut shell soot is obtained by pyrolysis method. The heating process using a furnace carried out
for 2 hours at 400 °C. Carbon dots produced by heating with 5 M HNO
3
for 12 hours at 100 °C, centrifuged
and dialysis. The yield of carbon dots was 87%. The FT-IR spectrum shows that the Carbon dots formed
produce OH group absorption at 3396 cm
-1
, absorption of C = C at wave number 1637 cm
-1
, absorption of
CO groups, and CH at wave number 1339 cm
-1
, and 835 cm
-1
. Analysis with Transmission Electron
Microscopy shows that carbon dots has an average diameter of 1.50 nm. Absorbance spectrum analysis
(UV-Vis) results in the appearance of new uptake showing the electron transition at a wavelength of 307 nm
and giving green fluorescence under UV light.
1 INTRODUCTION
Fluorescence nanoparticle material is receiving a lot
of attention due to its superiority and wide
application. One of the interesting properties of F-
NPs is the ability to fluency, so it is widely applied
as bioimaging. However, this material involves the
use of heavy metals such as Cd and Pb which are
known to have high toxicity so that their use are
limited. Based on this problem, another alternative is
used to replace the raw material from F-NPs, by
using carbon-based nanoparticles or known as
Carbon dots. Carbon dots is a material that has a
zero dimensional structure and is a product of
carbon nanotubes. Besides has properties that are
almost similar to F-NPs, the ability of fluorescence,
Carbon dots also have high solubility in water,
environmentally friendly, have low toxicity. and low
manufacturing costs (Baker & Baker, 2010). Carbon
sources that can be used as starting material for C-
dots include soot from burning candles (Liu et al.,
2007), nanocrystal cellulose (Marpongahtun et al.,
2018), burning plants (Tan et al., 2013) and coal (Ye
et al., 2013). Carbon dots can also be synthesized
from organic materials such as citric acid (Qu et al.,
2012) and ascorbic acid (Nisa, 2014).
Coconut shell is one of the materials that can be
used as charcoal, the residue produced when burning
charcoal is smoke. The process of the smoke taking
place in the combustion chamber is caused by the
fuel droplets collected into soot because the heating
is too large so that decomposition occurs.
Decomposition will cause carbon solid (soot)
(Arismunndar, 2002). Soot can be made by direct or
indirect heating in the pile or by the pyrolysis
method (Oladeji, 2010).
Various methods for the synthesis of Carbon dots
have been developed by scientists. In general, the
methods for carbon nanoparticle synthesis are based
on 2 approaches, the top-down and bottom-up
approaches. Bottom-up approach includes
electrothermal synthesis, microgear or ultrasonic,
hydrothermal, and acid oxidation (Li et al., 2012).
Carbon dots synthesis in this study uses a bottom-up
approach, through the HNO
3
acid oxidation method.
In addition to acid treatment by HNO
3
, the carbon
dots synthesis is also carried out by the purification
process by centrifugation, dialysis, and other
separation techniques (Li et al., 2012). All of these
processes are to obtain Carbon dots from a carbon
soot combustion. Based on the description above,
the author will conduct research on "Synthesis of
232
Marpongahtun, ., Irmayani, . and A., S.
Utilization of Coconut Waste as a Basic Material for Making Carbon Dots with Acid Oxidation Method.
DOI: 10.5220/0010139700002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 232-236
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

Carbon dots from coconut shell soot by acid
oxidation methods".
2 RESEARCH METHODS
2.1 Tools
The tools used in this study are glassware, FT-IR,
FE-SEM, TEM, UV lamps, UV-Visible spectra,
pyrolysis, and centrifugation.
2.2 Material
The materials used in this study are NaOH, HNO
3
(p), distilled water, Coconut Shell, and Dialysis
Membrane.
2.3 Research Procedure
2.3.1 Preparation of Coconut Shell
Coconut shell waste is cleaned from the remaining
coconut husks that still attached, cut into small
pieces and dried in the sun until the weight remains.
2.3.2 Making Soot from Coconut Shell
The dried coconut shell waste is put into a porcelain
cup, then heated using a furnace at 400 °C for 2
hours. The resulting soot was then analyzed by FE-
SEM.
2.3.2 Synthesis of Carbon Dots
Soot of carbon that has been obtained weighed as
much as 500 mg, put in a three neck flask. The
reflux assembly is arranged and a magnetic stir bar
is inserted. Then 100 mL of 5N HNO
3
was added
and refluxed at 100 °C for 12 hours. The result of
reflux is cooled at room temperature, after that it is
messed up using centrifugation at a speed of 4500
rpm for 30 minutes, then from the process will be
obtained 2 phases, the brown supernatant phase and
the solid phase in the form of black deposits, then
the two phases are separated and neutralized with
NaOH 5M (Li et al., 2012).
The neutralization is carried out until pH = 7,
after that the neutralization results are filtered using
filter paper and then dialyzed using a dialysis
membrane for 24 hours by continuing to replace the
aquadest water for 30 minutes 5 times at the
beginning of dialysis.
Dry carbon dots are characterized by UV lamps,
UV-Vis spectros, TEM, FE-SEM, and FT-IR.
3 RESULTS AND DISCUSSION
3.1 Results of Sample Preparation for
Coconut Shell Soot
The soot preparation is carried out using a furnace
tool. This preparation was carried out at 400 °C for 2
hours. The results of soot preparation can be seen in
Figure 1.
Figure 1: Results of coconut shell soot.
From 30 grams of coconut shell used will
obtained 5 grams of black carbon, 16.6% of the
initial weight of the coconut shell. Obtained Carbon
soot then analyzed using FT-IR to determine the
functional groups contained in coconut shell soot
and morphological analysis using FE-SEM.
3.2 Surface Analysis of Soot with
FE-SEM
Surface analysis of coconut shell soot using FE-
SEM can be seen in Figure 2.
The results shown in Figure 2 are the results of
coconut shell soot with a heating of 400 °C for 2
hours where the resulting soot has an average
surface diameter of 138.04 µm and appears to be
random piles.
Utilization of Coconut Waste as a Basic Material for Making Carbon Dots with Acid Oxidation Method
233

Figure 2: Results of morphological analysis of coconut
shell soot with enlargement 400 times.
3.3 Soot Carbon dots from Coconut
Shell with Acid Oxidation Method
Carbon dots solution obtained from the coconut shell
in the previous stage placed into a beaker glass then
evaporated to dry in an oven at 90 °C for 12 hours.
The C-dot results obtained can be seen in Figure 3.
Figure 3: Carbon dots from coconut shell soot.
From 0.5 grams of coconut shell soot 0.48 grams
of Carbon dots was obtained equal to 87% of the
initial weight of soot. Carbon dots from coconut
shell soot produce a yellowish brown color. The
oxidation reaction scheme can be seen in Figure 4.
Figure 4: Oxidation reaction scheme.
The addition of HNO
3
aims to disperse by
oxidizing molecules that have undergone
agglomeration. The reaction between carbon from
soot and 𝑁𝑂
ions causing electrons displacement
by the formation of complex compounds produced
Carbon dots are different from the base material.
Further interaction of complex compounds with
HNO
3
forms intercalation compounds (Savitskii,
2017).
3.4 Characterization
3.4.1 Analysis with UV Lamps
Fluorescence testing is carried out physically
through observation under a UV lamp. Carbon dots
produced through the acid oxidation method produce
flourescence under UV light as shown in Figure 5.
Figure 5: Carbon dots irridiation with UV lamps results:
(a) Negative control of coconut shell soot in visible light;
(b) Negative control of coconut shell soot under UV light;
(c) Carbon dots in visible light; (d) Carbon dots under UV
light.
The success of Carbon dots synthesis can be seen
from the fluorescence analysis using UV lamps.
Irradiation is carried out on a Carbon dots solution
with a concentration of 1000 ppm which can be seen
in Figure 5. These results show a green glow and in
accordance with the results reported by Ray et al.
(2009). Fluorescence occurs when electrons move
from the valence band to thes conduction band after
being excited by the excitation source in this case
UV light. Besides having good fluorescence
intensity, Carbon dots also have high solubility in
water (Liu et al., 2007). Figure 5 also shows that
138
,
04
µ
m
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
234
Carbon dots from coconut shell soot have higher
solubility compared to negative control. The
negative control made from a soot solution of pure
coconut shell before oxidation, dissolved in water at
concentration of 1000 ppm. The high solubility point
of Carbon dots is due to the successful functioning
of the Carbon dots surface. The functionalization
process will bring up functional groups such as
hydroxyl and carboxyl which will cause the surface
of Carbon dots to be negatively charged, so that
Carbon dots will be hydrophilic (Liu et al., 2007).
3.4.2 Absorbance Spectrum Analysis with
UV-Vis Spectrophotometry
The synthesis results of Carbon dots obtained were
then characterized using UV-Vis spectrophotometry.
Measurements were made at a wavelength of 200-
700 nm with wavelength interval of 100 nm.
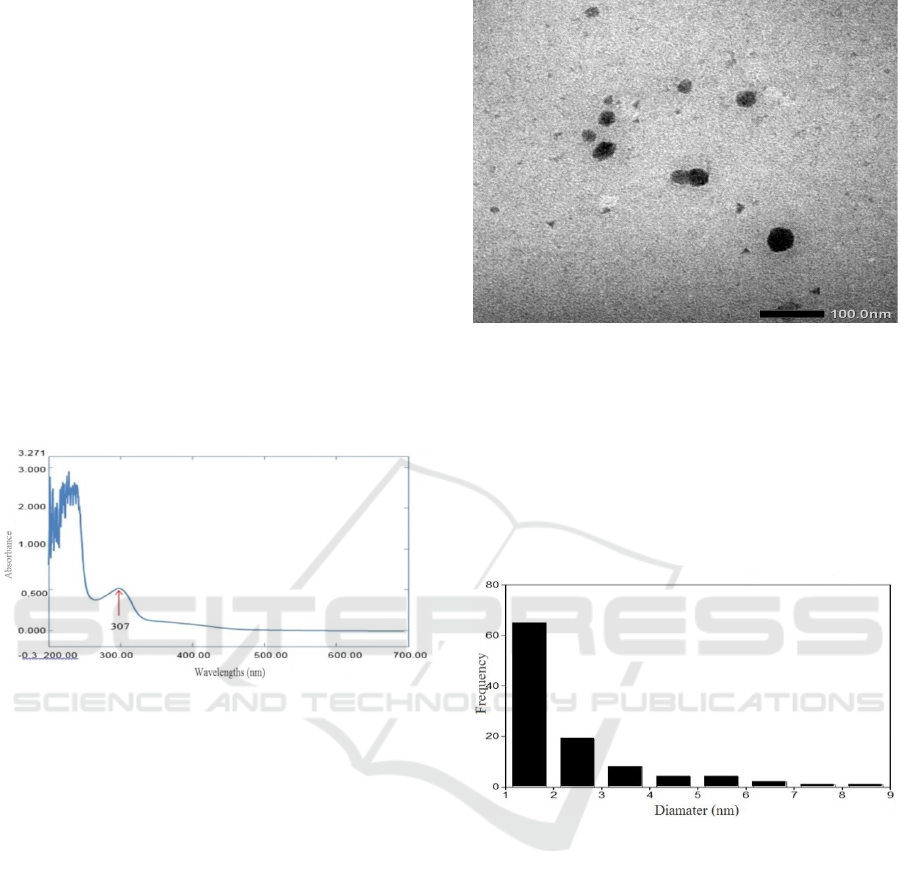
Figure 6: Absorbance curve with wavelengths from
coconut shell soot carbon dots.
Spectrum of the negative control Carbon dots of
coconut shell soot does not produce new absorption
at certain wavelengths (Figure 6), different from the
results of the Carbon dots spectrum of coconut shell
soot that produce new absorption at wavelengths of
307 nm with absorbance of 0.5040.
3.4.3 Analysis of C-dot Average Diameter by
using TEM
TEM analysis is used to find the average diameter of
the surface and to magnification of objects in a small
size. Carbon dots shaped like a ball in the form of
dots.
Figure 7: Results of TEM carbon dots with a dispersion of
400000 times.
Carbon dots is a new class of carbon
nanomaterials under 10 nm in size (Baker & Baker,
2010) based on the calculation of Carbon dots
diameter measurements that have been carried out
using the Image J application, obtained the average
diameter of C-dot soot in coconut shell is 1.50 nm.
The particle size distribution illustrated in Figure 8.
Figure 8: Result average diameter.
3.4.4 Analysis of the Function Group with
using FT-IR
Functional group analysis is performed using Opus
Alpha's Bruker FT-IR. Wave numbers obtained from
coconut shell soot and Carbon dots can be seen in
Figure 9.
Utilization of Coconut Waste as a Basic Material for Making Carbon Dots with Acid Oxidation Method
235
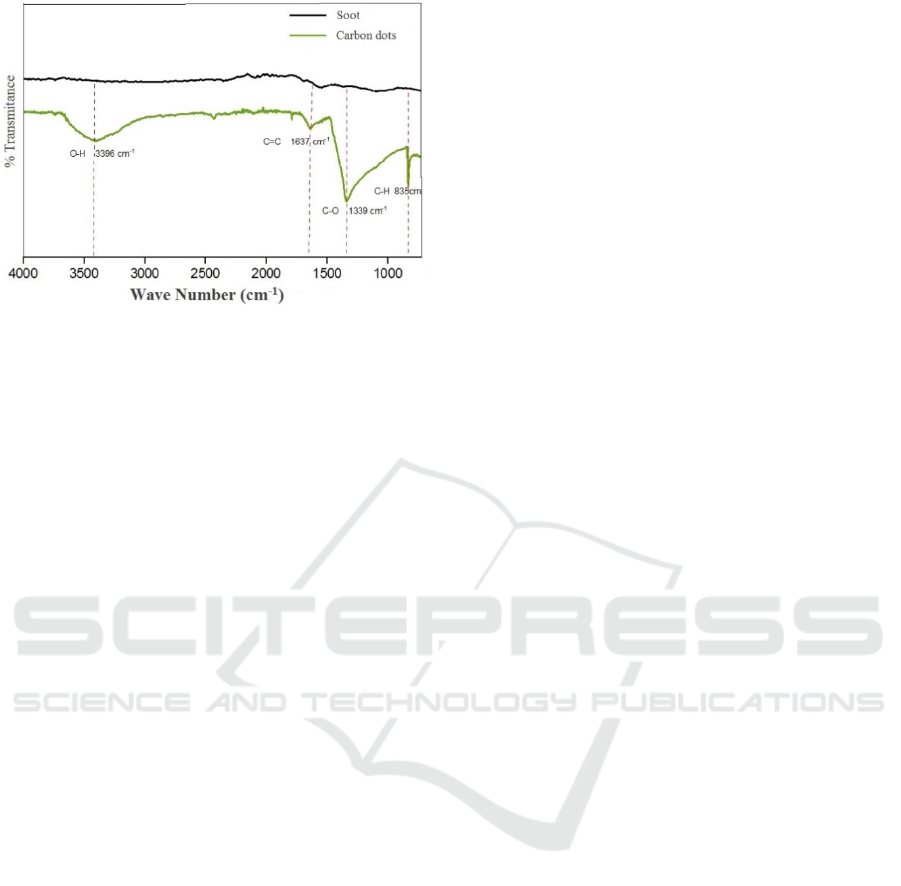
Figure 9: Wave numbers of coconut shell soot and C-dots
obtained using FT-IR.
In addition to measurements taken for Carbon
Dots samples, FT-IR measurements were also carried
out for samples of coconut shell soot. This can be
seen in Figure 9, and the interpretation of functional
groups seen in the picture explain that there are
differences in the absorption peaks between Carbon
dots samples and soot of coconut shells. Coconut
shell soot does not produce peak O-H absorption.
Carbon dots of coconut shell soot produce peak O-H
at wave number 3396 cm
-1
. These results explain that
in the structure of Carbon dots that have been
synthesized there are carboxylic functional groups.
This is consistent with the results on Carbon dots
coconut shell soot. Based on experiments reported by
Hu et al. (2014), coal also has a C = C uptake at a
wavelength of 1585 cm
-1
. These results are in
accordance with the Carbon dots that have been
synthesized, although experiencing a shift that is at
wave number 1637 cm
-1
and at wave number 1339
cm
-1
for C-O and 835 cm
-1
for C-H uptake.
4 CONCLUSIONS
Carbon dots can be made from coconut sheell soot
with acid oxidation method. The yield of Carbon
dots obtained was 87% of the initial weight of the
soot. The FT-IR spectrum shows that the Carbon
dots formed produce OH group absorption at 3396
cm
-1
, absorption of C = C at wave number 1637 cm
-
1
, absorption of CO groups, and CH at wave number
1339 cm
-1
, and 835 cm
-1
. Analysis with
Transmission Electron Microscopy shows that
carbon dots has an average diameter of 1.50 nm.
Absorbance spectrum analysis (UV-Vis) results in
the appearance of new uptake showing the electron
transition at a wavelength of 307 nm and giving
green fluorescence under UV light.
REFERENCES
Arismunndar, W. (2002). Motor Bakar Torak (5th ed.).
Institut Teknologi Bandung.
Baker, S. N., & Baker, G. A. (2010). Luminescent Carbon
Nanodots: Emergent Nanolights. Angewandte Chemie
International Edition, 49, 44. https://doi.org/10 .1002 /
anie. 200906623
Hu, C., Yu, C., Li, M. Y., Wang, X., Yang, J., Zhao, Z.,
Eychmuller, A., Sun, Y. P., & Qiu, J. (2014).
Chemically tailoring coal to fluorescent carbon dots
with tuned size and their capacity for Cu(II) detection.
Small, 10(23), 4926–4933.
Li, H., Kang, Z., Liu, Y., & Lee, S. T. (2012). Carbon
nanodots: synthesis, properties and applications.
https://doi.org/doi:10.1039/c2jm34690g
Liu, H., Ye, T., & Mao, C. (2007). Preparation Of
Flourescent Carbon Nanoparticles From Candle Soot.
Angewandte Chemie International Edition, 46, 6473–
6475. https://doi.org/10.1002/ anie.200701271
Marpongahtun, Gea, S., Muis, Y., Andriayani, Novita, T.,
& Piliang, A. F. (2018). Synthesis of Carbon Nanodots
from Cellulose Nanocrystals Oil Palm Empty Fruit by
Pyrolysis Method. Journal of Physics: Conference
Series, 1120(1), 1–6. https://doi.org/10.1088/1742-
6596/1120/1/012071
Nisa, A. K. (IPB). (2014). Sintesis nanopartikel karbon
berfluoresens.
Oladeji, J. (2010). Fuel characterization of briquettes
produced from corncob and rice husk resides. Pacific
Journal of Science and Technology, 11(1), 101–106.
Qu, S. G., Wang, X., Lu, Q., Lie, X., & Wang, L. (2012).
A Biocompatible Fluorescent Ink Based on Water-
Soluble Luminescent Carbon Nanodots. Angewandte
Chemie, 124, 1–5.
https://doi.org/10.1002/amge.201206791
Ray, S. C., Saha, A., Jana, N. R., & Sarkar, R. (2009).
Fluorescent Carbon Nanoparticle: Synthesis,
Characterization, and Bioimaging Application. J Phys
C, 113(43), 18548–18551.
https://doi.org/10.1021/jp905912n
Savitskii, D. P. (2017). Preparation and characterization of
colloidal dispersions of graphene-like structures from
different ranks of coals. In Ranliao Huaxue
Xuebao/Journal of Fuel Chemistry and Technology
(Vol. 45, Issue 8, pp. 897–907).
Tan, M., Zhang, L., Tang, R., Song, X., Li, Y., Wu, H.,
Wang, Y., Lv, G., Liu, W., & Ma, X. (2013).
Enhanced Photoluminescence and Characterization of
Multicolor Carbon Dots Using Plants Soot as a Carbon
Source. Talanta, 115, 950–956.
Ye, R., Xiang, C., Lin, J., Peng, Z., Huang, K., Yan, Z.,
Cook, N. P., Samuel, E. L. G., Hwang, C. C., Ruan,
G., Ceriotti, G., Raji, A. R. O., Martí, A. A., & Tour,
J. M. (2013). Coal as an abundant source of graphene
quantum dots. Nature Communications,
4, 1–7.
https://doi.org/10.1038/ncomms3943
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
236
