
Manufacture and Measurement of Graphene-based Supercapasitor
Electrodes and Characterization using Charging-discharging Method
Ivan Anggia Sihotang, Kerista Tarigan and Syahrul Humaidi
Department of Physics, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan, Indonesia
Keywords: Graphene, Activated Carbon, Epoxy Resin, Phosphoric Acid (H
3
PO
4
), Aluminum Plate, Supercapacitor.
Abstract: Supercapacitors or known as EDLCs (Electrochemically double-layer Capacitors) are electric double layers
separated by separators. Supercapacitors provide very high power density values, long repetition cycles and
have a higher repetition efficiency compared to batteries. This study aims to design a supercapacitor with a
material of 0.905 gram graphene powder, 2 gram activated carbon, epoxy resin (3 spoonfuls of spatula),
Phosphoric Acid (H
3
PO
4
) electrolyte solution, and aluminum plate with a size of 8 cm x 8 cm, 6 cm x 6 cm,
and 5 cm x 5 cm as collector media. The research was carried out in four stages, namely mixing graphene
powder and epoxy resin, coating graphene-epoxy resin powder on both plates, sowing activated carbon on
the surface of the plate, and combining the two plates into one supercapacitor section. The test results show
that with the charging-discharging method, the size of 8 cm x 8 cm which is carried out charging for 3
minutes can store a voltage of 1.65 Volt, on the size of 6 cm x 6 cm done charging for 3 minutes can store a
voltage of 1.44 Volt, and at a size of 5 cm x 5 cm by charging for 3 minutes can store a voltage of 1.05 Volt.
So it can be proven that the cross-sectional area of a supercapacitor greatly influences the value of the stored
voltage.
1 INTRODUCTION
The development of advancements in technology
now makes everyone need electronic devices that are
able to support their work to make it easier and more
practical. Therefore these electronic devices must
have a great ability to store energy. Large energy
storage is needed so that these electronic devices are
able to work optimally and are durable in order to
support the work to be more practical and efficient.
One of the energy storage that is commonly used is
the battery, the battery is used because it is more
practical and only disposable, but this is also a
disadvantage because it cannot last long in use, has
no economic value, produces waste that is harmful to
the environment, and the power is also stored tend to
be small. That is why lately people have begun to
turn to supercapacitors.
Supercapacitors or known as EDLCs
(Electrochemically double-layer Capacitors) are
electric double layers separated by separators.
Supercapacitors provide very high power density
values, long repetition cycles and have a higher
repetition efficiency compared to batteries. From a
technical point of view, supercapacitors have a
relatively large number of cycles (> 100000 cycles),
high energy density, large energy saving capacity,
simple principles and easy construction (Hyeok,
2001). Whereas in terms of user friendliness,
supercapacitors increase safety because there are no
corrosive materials and less toxic materials.
Supercapacitors collect the charge from the
absorption of electrostatic ions onto the surface of the
double layer electrode / electrolyte to the conduction
material at a specific surface area in this case,
activated carbon. The electrodes commonly used are
carbon and also metal plates. But what is often used
lately is carbon because metal plates have no
economic value and their ability as an electrode to
store a charge is relatively small. Therefore carbon is
more often used as an electrode in supercapacitors.
To increase the specific surface area, carbon is
activated so that its ability to increase the charge is
better.
Among carbon materials, graphene is the most
promising material as an electrode for energy storage
device applications because it has a high surface
area, is relatively inexpensive, has high electrical
conductivity. This material has an electron mobility
of 15,000 cm
2
/V•s, a thermal conductivity of 5,000
214
Anggia Sitohang, I., Tarigan, K. and Humaidi, S.
Manufacture and Measurement of Graphene-based Supercapasitor Electrodes and Characterization using Charging-discharging Method.
DOI: 10.5220/0010139300002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 214-218
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reser ved

Wm
-1
K
-1
. Graphene is an ideal material for
fabricating supercapacitors because it has a large
surface area of 2630 m
2
/g and intrinsic
electrochemical capacitance of ~ 21 mF/cm
2
. This
value is the maximum value for all carbon-based
materials (K & Carlen, 2000).
In this research, graphene-based supercapacitor
electrodes will be made, where graphene functions as
carbon material used in the process of making
supercapacitor electrodes.
2 MATERIALS AND METHODS
2.1 Material Used in Research
The materials used in this study are graphene
powder, activated carbon, epoxy resin, phosphoric
acid (H
3
PO
4
) electrolyte solution, aluminum plate,
and separator (tissue).
Graphene functions as a cathode electrode and a
double layer super capacitor anode that will receive
electrical energy from the collector and then store the
electric charge while after that the electrical charge is
wasted. In this study graphene powder used was
0.905 grams for each supercapacitor.
Activated carbon is used as an anode and cathode
just like graphene powder, and activated carbon
powder also functions as a store of electric charge
and a second layer after graphene to be given an
electrolyte solution. In this study active carbon
powder was used for 2 grams for each
supercapacitor.
Epoxy resins are used as an adhesive between
graphene powder, activated carbon and laminating
foil. in addition, epoxy resin also serves as a
protector so that the electrolyte liquid cannot touch
the collector laminating foil and also so that there is
no loss of capacitance that will make electrons move
to the collector. In this study epoxy resins used 3
spoonfuls of spatula for each supercapacitor.
Phosphoric Acid (H
3
PO
4
) electrolyte solution
functions as an electrolyte in the supercapacitor to be
dripped into a separator (tissue), where positive and
negative ions will react. Positive and negative ions
will move freely when before being given a voltage
and when given a positive and negative ion will be
attracted to the electrode.
This separator (tissue) functions as a separator
between anode and cathode mixed with an electrolyte
solution and also as a polarity bridge.
The aluminum plate is used as a collector
between the anode and the cathode, which is a double
layer or the right and left side of the supercapacitor
that will receive electrical energy and then be
delivered to the electrodes. The aluminum plate used
has a thickness of 0.2 mm with a size of 8 cm x 8 cm,
6 cm x 6 cm and 5 cm x 5 cm. The surface of the
aluminum plate must be clean so that the graphene
powder and activated carbon can be attached to the
surface of the aluminum plate.
2.2 Overall Research Procedure
This research procedure has several stages. First, the
aluminum plate is divided into two equal parts, each
measuring 8 cm x 8 cm, 6 cm x 6 cm and 5 cm x 5
cm. Then graphene is mixed with epoxy resin
powder with a ratio of 0.905 grams of graphene
powder and 3 tablespoons spatula epoxy resin, after
that it is stirred until evenly distributed. The results
of the mixture of graphene and epoxy resin were
immediately applied to both parts of the aluminum
plate, each of which had a specified size. Let stand
the results for 5 minutes, then sprinkle the activated
carbon powder on top of the aluminum plate evenly,
so that the activated carbon is attached to the
aluminum plate.
Second, unite the two parts of the aluminum plate
by placing a tissue between the two parts of the
aluminum plate while dripping with a solution of
Phosphoric Acid (H
3
PO
4
) electrolytes to the tissue in
an adequate ratio. Then press and clamped both sides
of the aluminum plate that was joined together so
that it sticks. Make a current collector on each part of
the electrode using a crocodile cable / clamp. After
that measured the voltage (V) stored and the length
of time with the charging-discharging method.
Figure 1: Three supercapacitors designed.
Double-layer electric capacitors or EDLCs are
based on the working principle of the dual electric
layers that form on the inter-surface layer between
activated carbon and electrolytes as dielectric. The
mechanism of absorption and desorption of ions on
both layers of activated carbon electrodes plays a role
in EDLCs charging and emptying. By applying
voltage to the facing electrodes the ions will be
attracted to the surface of the two electrodes and the
charging process will occur. Instead, ions will move
Manufacture and Measurement of Graphene-based Supercapasitor Electrodes and Characterization using Charging-discharging Method
215
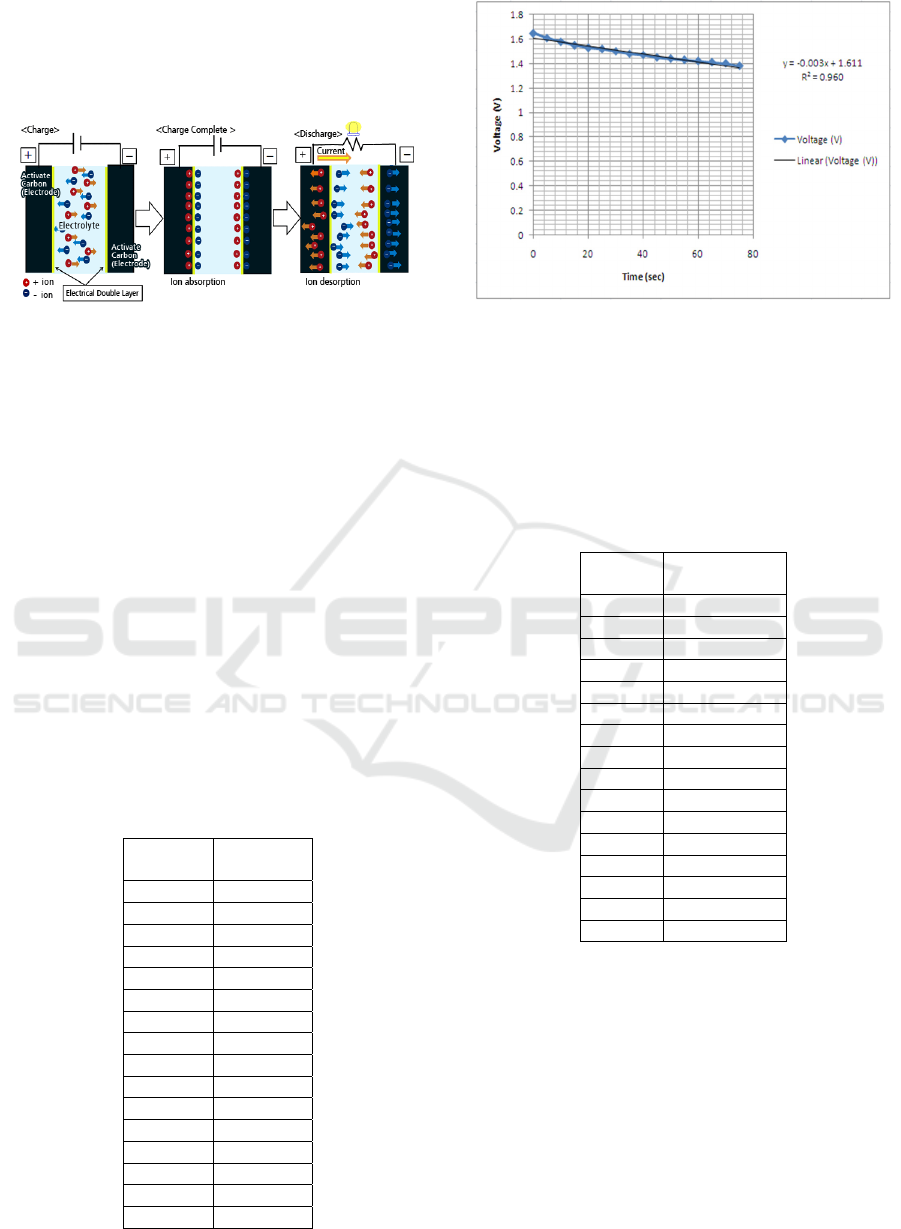
away when EDLCs is used or discharging (Murata
America Co. Ltd., 2011). The charging and
discharging process of EDLCs can be seen in Figure
2.
Figure 2: Scheme of charging and discharging process on
EDLCs (Murata co, Ltd, 2011).
3 RESULT AND DISCUSION
3.1 Charging-discharging Method
The results of the research making supercapacitors by
varying the cross-sectional area with a size of 8 cm x
8 cm, 6 cm x 6 cm, and 5 cm x 5 cm. Where in this
test using the charging-discharging method, charging
for 3 minutes with an input voltage (V) of 3 Volt.
3.1.1 Testing on a Supercapacitor with Size
of 8 Cm x 8 Cm
With the charging-discharging method, a storage
voltage of 1.65 Volt is obtained. And the time of
discharge can be seen in the table below.
Table 1: Discharge Time.
Time
(
sec
)
Voltage
(
V
)
0 1.65
5 1.61
10 1.58
15 1.55
20 1.53
25 1.52
30 1.50
35 1.48
40 1.47
45 1.45
50 1.44
55 1.43
60 1.42
65 1.41
70 1.40
75 1.38
Figure 3: Graph of discharge voltage vs time.
3.1.2 Testing on Supercapacitor with a Size
of 6 Cm x 6 Cm
With the charging-discharging method, a storage
voltage of 1.44 Volt is obtained. And the time of
discharge can be seen in the table below.
Table 2: Discharge Time.
Time
(sec)
Voltage
(V)
01.44
51.42
10 1.41
15 1.39
20 1.37
25 1.36
30 1.34
35 1.33
40 1.32
45 1.31
50 1.29
55 1.28
60 1.27
65 1.26
70 1.25
75 1.24
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
216

Figure 4: Graph of discharge voltage vs time.
3.1.3 Testing on Supercapacitors with a Size
of 5 Cm X 5 Cm
With the charging-discharging method, a storage
voltage of 1.05 Volt is obtained. And the time of
discharge can be seen in the table below.
Table 3: Discharge Time.
Time
(s)
Voltage
(V)
0 1.05
5 1.04
10 1.03
15 1.02
20 1.02
25 1.01
30 1.00
35 0.99
40 0.98
45 0.97
50 0.97
55 0.96
60 0.95
65 0.94
70 0.93
75 0.92
Figure 5: Graph of discharge voltage vs time.
3.2 Capacitance Testing of
Supercapacitor
This test is carried out to find out what is the value of
capacitance in each supercapacitor. Where the plate
cross-sectional area is known, the distance between
the two plates and the dielectric constant value of the
material. By using the equation below.
𝐶 𝜀
o
𝜀
r
(3.1)
3.2.1 Capacitance Testing of Supercapacitors
with a Size of 8 cm x 8 cm
Determine the cross-sectional area of the
supercapsitor using the equation below.
A = s x s (3.2)
Then obtained:
A = 8 cm x 8 cm
A = 64 cm
2
Next determine the capacitance value using equation
3.1. where for the similar
𝜀
r
separator value which is
a polymer material is assumed to be 10,000 through
previous research. So we get the results:
𝐶 𝜀
o
𝜀
r
𝐶
8.854 𝑥 10
𝐹. 𝑚
𝑥 10000 𝑥
64 𝑐𝑚
1 𝑚𝑚
𝐶 0,57 𝑥 10
𝐹
3.2.2 Capacitance Testing on
Supercapacitors with Size 6 cm x 6 cm
Determining the cross-sectional area of the
supercapsitor by using equation 3.2 we get:
A = s x s
A = 6 cm x 6 cm
A = 36 cm
2
Next determine the capacitance value using equation
3.1 so that the results are obtained:
𝐶 𝜀
o
𝜀
r
𝐶
8.854 𝑥 10
𝐹. 𝑚
𝑥 10000 𝑥
36 𝑐𝑚
1 𝑚𝑚
𝐶 0,319 𝑥 10
𝐹
Manufacture and Measurement of Graphene-based Supercapasitor Electrodes and Characterization using Charging-discharging Method
217

3.2.3 Capacitance Testing on
Supercapacitors with a Size of 5 Cm X
5 Cm
Determining the cross-sectional area of the
supercapsitor by using equation 3.2 we get:
A = s x s
A = 5 cm x 5 cm
A = 25 cm
2
Next determine the capacitance value using equation
3.1 so that the results are obtained:
𝐶 𝜀
o
𝜀
r
𝐶
8.854 𝑥 10
𝐹. 𝑚
𝑥 10000 𝑥
25 𝑐𝑚
1 𝑚𝑚
𝐶 0,221 𝑥 10
𝐹
4 CONCLUSIONS
Manufacture and Measurement of Graphene-Based
Supercapasitor Electrodes and Characterization
Using Charging-Discharging Method have been
done. The results show that with the charging-
discharging method, a supercapacitor with a size of 8
cm x 8 cm, 6 cm x 6 cm, and 5 cm x 5 cm after
charging for 3 minutes with a 3 volt input voltage
will obtain the stored voltage in a row of 1.65 volts,
1.44 volts, and 1.05 volts. And by varying the cross-
sectional area of the supercapacitor, the capacitance
value can also be determined using a predetermined
equation. So that it can be proven that the cross-
sectional area of a supercapacitor greatly affects the
value of capacitance and the amount of voltage
stored.
REFERENCES
Hyeok, A. K. (2001). Electrochemical Properties Of High
Power Supercapacitors Using SingleWalled Carbon
nanotube Electrodes. Advanced Functional Materials,
11, 387–392.
K, R., & Carlen, M. (2000). Electrochim. Acta, 45, 2483.
Murata America Co. Ltd. (2011). High Performance
Electrical Double Layer Capacitor. Smyrna: Murata
Electronics.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
218
