
Flavonoid Compound from Rambutan Bark
(Nephelium lappaceum L.)
Helmina Br Sembiring
and Mesy Jelisa
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan, Indonesia
Keywords: Isolation, Nephelium lappaceum L., Flavonoids, Flavonol.
Abstract: Flavonoids included polyphenol compounds that are found in many plants which are important compounds
in human food.The flavonoid group, including flavanone, flavone, dihydroflavonol, catechin, flavonol,
flavan-3-ols, isoflavones, auron, anthocyanidins, proanthocyanidins and chalcones, has a general structure of
C6-C3-C6. The aims of this study is to isolate and identify the group of flavonoid compound contained in
rambutanbark (Nephelium lappaceum L.). Rambutan bark powder was extracted maceration with methanol.
Methanol extract was dissolved with ethyl acetate repeatedly until the solution was negative flavonoids. Ethyl
acetate extract was dissolved with methanol and partitioned with n-hexane. The methanol extract which is a
total of flavonoids separated by column chromatography using chloroform: methanol (90:10; 80:20 and 70:30
(v/v)). The isolates obtained were purified by preparative thin layer chromatography and producing flavonoid
glycoside, yellow amorphous with Rf value of 0.25 using the chloroform: ethyl acetate eluent (50:50) v/v.
The pure isolate obtained were analyzed by UV-Visible Spectropometer, FT-IR, and H-NMR. Based on the
interpretation of spectroscopic data, the flavonoid compound isolated from rambutan bark wasflavonol group.
1 INTRODUCTION
Rambutan (Nephelium lappaceum L.) is an evergreen
tree (Sukmandari et al., 2017), a plant that identical
with Southeast Asian countries, in some areas of
Indonesia (Wahini et al., 2018). The dried rambutan
fruit peel is used in traditional medicine, cooking and
in the manufacture of soap. The roots bark and
rambutan leaves have various uses in medicine and in
the production of dye (Suganthi & Josephine, 2016).
Rambutan fruit peel contains flavonoids, tannins and
saponins (Hariana, 2006).
Flavonoids are the most extensive groups of
phenolics Flavonoids are secondary with low
molecular weight that have bioactivity (Weston &
Mathesiu, 2013). Flavonoids are polyphenol
compounds composed of 15 carbon atoms, with two
aromatic rings connected by a bridge consisting of
three carbon atoms (Crozier et al., 2006). The
flavonoid group, including flavanone, flavone,
dihydroflavonol, catechin, flavonol, flavan-3-ols,
isoflavones, auron, anthocyanidins,
proanthocyanidins and chalcones, has a general
structure of C6-C3-C6 (Rosa et al., 2010).
Flavonoids have a positive effect on human and
animal health. Flavonoids are widely used as a
therapy for disease and chemoprevention (Panche et
al., 2016). Bioactivities of these phenolic,
polyphenolic acids or essential oil are potential as
new leads for the development of pharmaceutical
(Saranya et al., 2017), antibacterial (Sembiring et al.,
2019 )and agricultural products to improve human
health and nutrition (Khadem & Marles, 2010)
.Flavonoids are able to treat diseases, such as cancer
and heart disease (Zhang et al., 2015) can be used to
protect the human body from free radicals (Megawati
et al., 2015), (Molyneux, 2004) and can reduce the
risk of cancer and inflammation (Kumar & Pandey,
2013).
Pangalinan et al., (2012) mentioned that rambutan
bark are effective as antifungal against Candida
albicans. The purpose of this study was to isolated
and identified the flavonoid group contained in the
bark of the rambutan (N. Lappaceum L.). Flavonoid
compounds were isolated by maceration extraction
and column chromatography methods. Flavonoid
compound identification was carried out by FT-IT,
UV-Visible and HNMR spectroscopy.
Br Sembiring, H. and Jelisa, M.
Flavonoid Compound from Rambutan Bark (Nephelium lappaceum L.).
DOI: 10.5220/0010139100002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 205-208
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
205

2 METHODS
2.1 Material
Rambutan bark was obtained from Gelugur Rimbun
Village, Pancur Batu District, Deli Serdang, North
Sumatra, Indonesia. Identification of plant was done
at Herbarium Medanense (MEDA) Universitas
Sumatera Utara. All chemicals used such as Silica Gel
(70 – 230 mesh), for column chromatography, FeCl
3
,
NaOH, Serbuk Mg, HCl
(p)
, H
2
SO
4(p)
, kloroform,
silika gel 60 F
254
for thin layer chromatography, KLT
Preparative 60 F
254
and methanol were from E Merck,
methanol and ethyl acetate as solvent were distilled
before used (Saldanha et al., 2013).
2.2 Instrument
The
1
H NMR spectra were recorded with a (Agilent
500 MHz, Frekuensi 500 MHz), spectrometer
instrument with CD
3
OD as a solvent and TMS as an
internal standard and chemicalshifts are given in δ
(ppm). IR spectra were recorded on FT-IR (type
Mb3000, 485 – 8500 cm
-1
), UV spectra were recorded
on Spektrofotometer UV-Visible (Type UV – 1800
Shimadzu, 190 – 1100 nm), evaporation of solvents
with rotary evaporator (Heidolph), spotting
monitoring with lights of UV(254nm/356nm, UVGL
58).
2.3 Procedure
Isolation flavonoid compounds were done based on
(Megawati et al., 2015) and (Hostettmann et al.,
1995) with a slight modification. Rambutan bark
powder (1900 g) was macerated for 2 days using 11
L methanol (until all samples were submerged with
methanol). Maserat is accommodated and the solvent
is evaporated with a rotary evaporator and dried with
a water bath until a dry methanol extract was
obtained. Dry methanol extract was re-extracted with
ethyl acetate to separate tannins. The filtrate obtained
was evaporated with a rotary evaporator and water
bath until all the ethyl acetate solvent evaporated.
Ethyl acetate extract was redissolved with methanol
and repartitioned with n-hexane until the n-hexane
layer was colorless. The methanol layer was re-
concentrated with a rotary evaporator and dried with
a water bath to obtain a dry extract of methanol.
The dried methanol extract (5g) was added to the
column chromatographic containing silica gel slurry,
eluted with chloroform: methanol (90:10; 80:20;
70:30 v / v) slowly. The isolates were collected in
vials every 10 ml, then analyzed with TLC. The
fractions that have the same Rf value are combined.
Fractions of 41-85 have the same Rf value, combined
then purified with preparative TLC with cloroform:
etil asetat (40:60) (v/v), ) and produced one band spot
at the Rf 0.25. The band spot was crushed, eluted with
metanol: etil asetat (1:1)
v
/
v
, evaporated to obtain 7.9
mg pure isolate in the form of yellow amorphous. The
pure isolate was identification by UV-Vis, FT-IR and
1
H-NMR spectroscopy.
3 RESULTS AND DISCUSSION
The sample used in this study was the rambutan bark,
Nephelium lappaceum L. (Figure 1A), family
Sapindaceae. The pure isolate isolated from the bark
rambutanis a yellow amorphous (Figure 1B) and
identified by using UV-Vis, FT-IR and
1
H-NMR
spectroscopic analysis.
B
A
Figure 1: A. Rambutan plant; B. isolate.
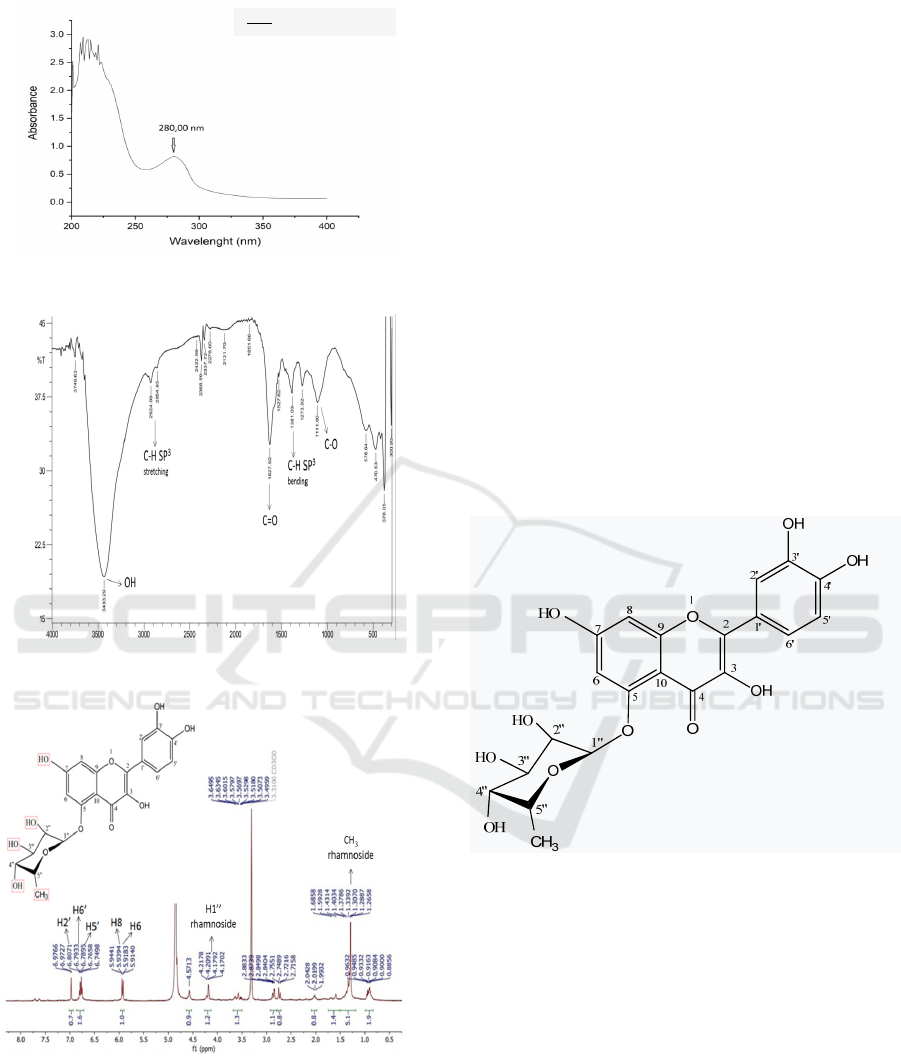
The UV-Visible (CH
3
OH) spectra was shown in
Figure 2. Based on the spectra, the isolate isolated
from rambutan bark was fllavonol group, because
presence of spectraat λ
max
280,00 nm. Absorption
band II at maximum wavelength (max λ) 280.00 nm
is a flavonoid of the flavonol group (Andersen &
Markhan, 2006). FT-IR spectra of pure isolated was
shown in Figure 3. The FT-IR spectra for pure
isolates showed (KBr, ν max, cm
-1
) 3433.29(O-H),
2854,65 and 2924,09 (C-H sp
3
stretching),1627.92
(C=O),1527.62(C=C), 1381.03 (C-Hsp
3
bending) and
1273,02(C-O).All of these vibrations are common
vibrations found in flavonoid compounds. The
stretching vibration of the C-H sp
2
bond is not
detected because it overlaps with the broad vibration
of the O-H bond (Pavia et al., 2001).
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
206
RambutanBark
Figure 2: The UV-Visiblespectra of isolate.
Figure 3: The FT-IR Spectra of pure isolate.
Figure 4: The
1
H NMR spectra of pure isolate.
1
H NMR spectra of pure isolate wasdepected in
Figure 4. Based on 1H NMR spectra (Methanol-D6,
500 MHz, (ppm)), chemical shift (δ (ppm)): δ 5.914
and 5.918 (1H, d, H-6), δ 5.939 and 5.944 (1H, d, H-
8), δ 6.750 and 6.766 (1H, d, H-5’), δ 6.790 and 6.807
(1H, d, H-6’) and δ 6.972 and δ 6.977 (1H, d, H-2’),
)), the isolate had five aromatic protons and eight
rhamnose protons. The type and number of protons in
this isolate are the same as the type and number of
protons of quercetin compounds reported by Huang
et al.,(2013) But it has a slight difference in chemical
shift and splitting pattern, due to differences in the
frequency of the spectroscopic used. Substituent -OH
bind toC-3, C-7, C-3’, dan C-4’. However, C-5 has a
glycoside bond because no -OH peaks are found at a
chemical shift of 12.22 ppm (Claramunt et al., 2006).
Rhamnose is thought to bind to flavonoids because of
the presence of peaks δ (ppm) 4,1702 dan δ 4,1792
(1H, d, H-1’’), δ 3,6495 (4H, m, H-2’’,H-3’’,H4’’,H-
5’’) and δ 1.289 and 1.307 (3H, d, -
CH
3
rhamnoside)ofrhamnose. The chemical shift of
rhamnose protons are similar to the chemical shift of
rhamnoseprotons reported by Plazonić et al, 2009)
Based on interpretation data of the UV-Visible,
FT-IR and 1H-NMR spectra, the flavonoid compound
isolated from rambutan bark was flavonol group
bound torhamnose at C-5with the structure shown as
in Figure5.
Figure 5: Structure of isolate.
4 CONCLUSIONS
Isolate obtained from 1900 g rambutan bark (N.
lappaceum L.) is a flavonoid 7.9 mg yellow
amorphous with an Rf value of 0.25 using the
chloroform: ethyl acetate eluent (50:50) v / v. Based
on the spectra and interpretation data the flavonoid
compound isolated from rambutan bark was flavonol
group.
Flavonoid Compound from Rambutan Bark (Nephelium lappaceum L.)
207

ACKNOWLEDGEMENTS
We would like to thank to Herbarium medananse
(MEDA), Laboratory of Natural Sciences Chemistry
Faculty of mathematical and Science Universitas
Sumatera Utara. We would also like to thank to Roch
Fitni for analysis of Spektrofotometer UV Visible and
FT-IR LPPT UGM Jl. Kaliurang Km. 4 Sekip Utara
Yogyakarta andand Elvira Hermawati for the analysis
of
1
H-NMR, Laboratory of Organic Chemistry, ITB
Bandung.
REFERENCES
Andersen, Q. ., & Markhan, K. R. (2006). Flavonoids
Chemistry. Taylor and Francis.
Claramunt, R. ., Lopez, C., Marıa, M. D. ., Sanz, D., &
Elguero, J. (2006). The use of NMR spectroscopy to
study tautomerism. Progress in Nuclear Magnetic
Resonance Spectroscopy, 49, 169–206.
Crozier, A., CliffordMN, & H, A. (2006). Plant Secondary
Metabolites: Structure and Role in Human Diet.
Blackwell Publishing Ltd.
Hariana, H. . (2006). Tumbuhan obat dan khasiatnya.
Penebar Swadaya.
Hostettmann, K., Hostettmann, M., & Marston, A. (1995).
Cara Kromatografi Preparatif, Penggunaan Pada
Senyawa Bahan Alam. ITB.
Huang, W., Wan, C., & Zhou, S. (2013). Quercetin - A
Flavonoid Compound from Sarcopyramis bodinierivar
delicate with Potential Apoptotic Activity in HepG2
Liver Cancer Cells. Trop J Pharm Res., 12(4), 529–533.
Khadem, S., & Marles, R. . (2010). Monocyclic Phenolic
Acids; Hydroxy- and Polyhydroxybenzoic Acids:
Occurrence and Recent Bioactivity Studies.
Review.Molecules, 15, 7985–8005.
Kumar, S., & Pandey, A. K. (2013). Review Article
Chemistry and Biological Activities of Flavonoids: An
Overview. The Scientific World Journal., 1-16.
Megawati, Saepudin, E., Hanafi, M., Darmawan, A., &
Lotulung, P. D. . (2015). Identification and Bioactivity
Studies of Flavonoid Compounds from
Macarangahispida (Blume) Mull.Arg.Makara. J. Sci.,
19(3), 96–100.
Molyneux, P. (2004). The Use of the Stable Free Radical
Diphenyl picrylhydrazyl (DPPH) for Estimating
Antioxidant Activity, Songklanakarin. J. Sci. Technol,
26(2), 211–219.
Panche, A. ., Diwan, A. ., & Chandra, S. . (2016). REVIEW
ARTICLE Flavonoids: an overview. Journal of
Nutritional Science, 5, 1–15.
Pangalinan, F. ., Kojong, N., & Yamlean, P. V. . (2012). Uji
aktivitas Antijamur Ekstrak Etanol Kulit Batang
Rambutan Terhadap Jamur Candida albicans Secara In
Vitro. Jurnal Farmasi, 1(1), 1-12.
Pavia, D. ., Lampman, G. ., & Kriz, G. . (2001).
Introduction to Spectroscopy (Third Edit). Thomson
Learning Inc. United States of America.
Rosa, d. l, L.A, Alvarez-Parrilla, E., & Gonzalez-Aguilar,
G. . (2010). Fruit and Vegetable Phytochemicals-
Chemistry, Nutritional Value, and Stability, (1st ed.). .;
Wiley-Blackwell: Ames, IA,.
Saldanha, L. L., Vilegas, W., & Dokkedal, A. L. (2013).
Characterization of flavonoids and phenolic acids in
Myrcia bella cambess. Using FIA-ESI-IT-MSn and
HPLC-PAD-ESI-IT-MS combined with NMR.
Molecules, 18(7), 8402–8416.
https://doi.org/10.3390/molecules18078402
Saranya, D., Sekar, J., & Adaikala, R. . (2017). Assessment
of antioxidant activities, phenol and flavonoid contents
of different extracts of leaves, bark and root from the
Abutilon indicum(L.) sweet. Asian J Pharm Clin Res,
10, 88–94.
Sembiring, H. ., Sihotang, H., & Tampubolon, A. . (2019).
Antibacterial Activities of Rough Lemon (Citrus
jambhiriLush) Rind Essential Oil. Journal of Chemical
Natural Resources., 1(1), 12–18.
Suganthi, A., & Josephine, R. M. (2016). Nephelium
lappaceum (L.): An overview. International Journal of
Pharmaceutical Science and Research., 1(5), 36–39.
Sukmandari, L. N. ., Dash, G. ., Jusof, W. H. ., & Hanaf, M.
(2017). A Review on Nephelium lappaceumL.
Research J. Pharm. and Tech, 10(8), 1–9.
Wahini, M., Miranti, M. ., Lukitasari, F., & Novela, L.
(2018). Rambutan Seed (Nephelium lappaceum L.)
Optimization as Raw Material of High Nutrition Value
Processed Food. IOP Conf. Ser.: Mater. Sci. Eng.,
306(012089).
Weston, L. ., & Mathesiu, U. (2013). Flavonoids: Their
Structure, Biosynthesis and Role in the Rhizosphere,
Including Allelopathy. J ChemEcol, 39, 283–297.
Zhang, Y., Gan, R., Li, S., Zhou, Y., Li, A., Xu, D., & L,
H. (2015). Review Antioxidant Phytochemicals for the
Prevention and Treatment of Chronic Diseases.
Molecules, 20, 21138–21156.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
208
