
Effectivity Test of Loquat (Eriobotrya japonica (Thunb.) Lindl.)
Leaves Extract on the Incision Wound Healing in Mice
(Mus musculus L.)
Emita Sabri and Raysa Zahra
Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan, Indonesia
Keywords: Eriobotrya japonica (Thunb.) Lindl., Incision Wound, Mice, Wound Healing.
Abstract: The wound healing process is divided into four phases; 1) coagulation 2) inflammatory 3) proliferation 4)
remodelling. Loquat (Eriobotrya japonica (Thunb.) Lindl.) leaf contains triterpenoid, flavonoid, alkaloid,
and tannin as anti-inflammation, antibacterial, anti-allergic that can accelerate the wound healing process.
The aims of this research were to find out the effectiveness of ethanol extract of loquat leaves and to
examine the histological appearance of wound healing process on mice’s skin in day 14
th
. This research
used 25 male mice (Mus musculus L.) that were divided into five different treatment groups. The treatment
groups were treated with two control groups; povidone-iodine (K+) and without treatment (K-) and loquat
leaves ethanol extract with three concentration of 15% (PI), 30% (PII), and 45% (PIII). Incision wound was
made into 1 cm length, the ointments were applied onto the wound and observed for twice a day in 14 days.
Histological preparation was made to calculate epithelial thickness, fibroblasts, and lymphocytes. The data
were analyzed statistically using SPSS. The result of this research showed significant difference of average
time span of wound healing of each group's, K-, K+, PI, PII, and PIII was subsequently 8 days, 9.2 days, 7.6
days, 8.4 days, and 9.4 days, with p=0.048 (p<0.05). Histological observations showed the average of
epithelial thickness, fibroblasts, and lymphocytes in the treatment group PI is higher than the other treatment
groups. In conclusion, loquat leaves ethanol extract with concentration 15% (PI) was the most effective to
accelerate the wound healing process.
1 INTRODUCTION
A wound is defined as damage or disturbance that
occurs in the structure and function of normal
anatomy tissue caused by a defense, chemical or
trauma. Incision wound is a wound that can be
healed properly if complications do not occur. The
criteria for an incision wound are new, sudden and
fast to healed (Perdanakusuma, 2007). Wound
healing is a natural process that occurs after an
injury, predictable progression of steps used by the
body to resolve impaired tissue integrity (Szycher
and Lee, 1992).
Wound healing is a complex process that
requires a series of steps, some of the steps are
granulation, collagen maturation, and scar
formation. (Zheng and Qin, 2007) which divided
into four phases: 1) coagulation and hemostatis; 2)
inflammation; 3) proliferation; 4) remodeling of a
wound (Velnar et al., 2009).
The hemostatic phase will begin the healing
process where the skin slashed then blood clots
containing fibrin and blood cells fill a narrow space
at the edge of the incision. Followed by an
inflammatory process, which starts 24 hours after
the incision occurs (Ross and Pawlina, 2011). The
inflammatory phase is the phase of the formation of
immunity such as leukocytes which plays an
important role for the initial inflammatory response
after the occurrence of wounds that can prevent the
entry of microorganisms to avoid acute injuries
(Robson et al., 2001). Proliferative phase is
characterized by the formation of granulation tissue
within the wound bed, composed of new capillary
network, fibroblast, and macrophages in a loose
arrangement of supporting structure (Prasettyono,
2009).
Indonesia has many plants that have various
benefits, one of them as medicine. The use of plants
as traditional medicine is widely used as an
162
Sabri, E. and Zahra, R.
Effectivity Test of Loquat (Eriobotrya japonica (Thunb.) Lindl.) Leaves Extract on the Incision Wound Healing in Mice (Mus musculus L.).
DOI: 10.5220/0010138100002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 162-170
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

alternative for healing because it is easy to obtain,
use and safer in terms of side effects.
The loquat (Eriobotrya japonica (Thunb.) Lindl.)
leaves have been used as a component in Chinese
medicine. Loquat is grown well in Kabanjahe,
Kabupaten Karo, North Sumatera, Indonesia, has
potential genetic compares with another loquat. The
fruit is large, has a very sweet taste, and becomes
multifunction plants. The leaves and the seeds are
containing bioactive compounds that are commonly
used for Karo traditional medicine to cure diabetic
disease and for expectorant to relieve cough
(Nurwahyuni et al., 2017).
Loquat leaves contain various triterpenes,
sesquiterpenes, flavonoids, tannins, and
megastigmane glycosides and these compounds have
been reported as anti-inflammation, anti-viral,
antioxidant, anti-mutagenic, and anti-tumor (Banno
et al., 2005), anti-bacteria and anti-allergic (Tan et
al., 2017). In a study conducted by Zhang et al.
(2015) founded 19 secondary metabolite contents in
loquat leaves for various bioactivities, such as anti-
inflammatory, antioxidant, and anti-cancer
properties. Mong these 19 ingredients, there are 15
components, most of which are classified as
triterpenoids and flavonoids that show
antiinflammatory activity.
Various pharmacological and biology reports
showed that the medicinal value of plants lies in
bioactive phytochemical constituents that produce
definite physiological action on the human body.
The herbal extracts promote fast wound healing than
control and non medicated group in different in vivo
studies, the process is promoted by several herbal
extracts, which are composed of active agents like
triterpenes, alkaloids, flavonoids, tannins, saponins,
anthraquinones, and other biomolecules (Thakur et
al., 2011). According to the research of Kimura et al.
(2008), Lee et al. (2006) and Liu et al. (2008) that
asiaticoside, a triterpenoid component can accelerate
the wound healing process in mice by increasing
antioxidant activity, collagen synthesis, and
angiogenesis. Two triterpenes compounds,
asiaticosides and madessicosides, showed better
result wound healing pattern in a view of
histological examination (Wu et al., 2012).
From the various research data above, it shows
that the loquat leaves have the potential to heal the
wounds due to the content of secondary metabolites
which have anti-inflammatory, antibacterial,
antiallergic and antioxidant effects that an accelerate
wound healing. In this case, a study was conducted
to test whether ethanol extracts of loquat leaves can
accelerate wound healing in mice (Mus musculus).
2 MATERIALS AND METHODS
2.1 Grouping and Dosing of Animals
This study was taken in Laboratory Animal
Structure and Physiology, Faculty of Mathematics
and Natural Science, Sumatera Utara University.
Twenty-five male mice (Mus musculus L.) were
used for the study. Mice weighed 25-30 grams and
aged 8-10 weeks. Mice were housed in cages and
provided with adlibitum pellet and water. The study
protocol was approved by the Ethics committee of
the Faculty of Mathematics and Natural Science,
Sumatera Utara University, AREC. Mice were
divided into five groups. The treatment groups were
treated with two control groups; povidone-iodine
(K+) and without treatment (K-) and loquat leaves
ethanol extract with three concentration of 15% (PI),
30% (PII), and 45% (PIII).
2.2 Plant Extraction
Loquat leaves were dried for 3 three weeks under the
shade. The dried leaves were grinded to coarse
powder. The powder was macerated with 70%
ethanol as a solvent in 6 hours with occasional
shaking and stirring, then soaked for 18 hours. The
extract was then filtered. The maceration process is
repeated once again. The filtrate was then combined
and evaporated in a rotary evaporator at 40°C and
concentrated in water bath until a thick extract is
obtained (Kemenkes RI, 2013).
2.3 Ointment Formulation
Simple ointment of the loquat leaves ethanol extract
with vaseline was prepared following the formula
(Table 1) based on Kusumawardhani et al. (2015):
L
𝑥 100 % (1)
L : Ointment concentration (%)
a : Loquat leaves ethanol extract (gram)
b : Ointment (50 gram)
Table 1: Ointment formulation.
Ingredients
Ointment (gram)
15% 30% 45%
Vaseline 42,5 35 27,5
Ethanol extract
of loquat leaves
7,5 15 22,5
Effectivity Test of Loquat (Eriobotrya japonica (Thunb.) Lindl.) Leaves Extract on the Incision Wound Healing in Mice (Mus musculus L.)
163

2.4 Incision Wound Model
After acclimatization in a week, mice were
anesthetized in the same manner to reducing the
pain. The dorsal of each mouse was then shaved and
a 1 cm long incision was made through the skin. The
scalpel was marked on the tip for the depth of the
wound. Mice were treated under grouping dosing
section and the ointment formulation as described.
Treatments were started from day 1
st
until day 14
th
.
Observed in twice a day, at 08.00 AM and 05.00
PM.
2.5 Histological Assessment
The skin samples were taken in day 14th.
Histological assessment using paraffin methods and
stained by Hematoxylin-Eosin stain (Suntoro, 1983).
2.6 Data Parameters
Data parameters were divided into macroscopically
and microscopically. Macroscopical data was the
average time span of wound healing in each group.
Microscopical data was average of epithelial
thickness, fibroblasts, and lymphocytes on day 14
th
in skin mice. Microscopical examination used
OptiLab Microscope Camera with magnification
100x for epithelial thickness and 400x for
calculating fibroblast and lymphocyte cells.
The data
were analyzed using SPSS version 22.0.
3 RESULTS
3.1 The Average Time Span of Wound
Healing
The effect of ethanol extract of loquat leaves on
wound healing day was marked by the wound
having healed. Data on the average time span of
wound healing day of mice can be seen in Table 2.
Table 2: Average time span of wound healing.
Groups Average time span ± SD
K
- 8,0
ab
± 0,70
K+ 9,2
b
± 0,83
PI 7,4
a
± 1,14
PII 8,4
ab
± 1,14
PIII 9,4
b
± 1,14
Data were tested with One Way ANOVA and
obtained significant results of 0.028 (p <0.05),
followed by Duncan test and the results showed that
the treatment group of ethanol extract of leaves of
15% concentration (PI) was the most potent
treatment on wound healing seen from average time
span of wound healing in mice.
Wounds treated with a concentration of 15% (PI)
provide a faster healing effect when compared to
other treatments and treatment with a concentration
of 45% (PIII) provides the longest healing effect of
all treatments. Normal wound healing can take place
naturally without assistance such as healing that
occurs in negative control treatments (K-), but herb
extracts such as at a concentration of 15% (PI)
loquat leaves will help in accelerating wound
healing because it contains secondary metabolites
that have an effect anti-inflammatory and
antibacterial properties such as terpenoids and
alkaloids.
Accordance to Rahman et al. (2013) said that the
speed of the wound healing process can be
influenced by the compound of secondary
metabolites such as terpenoid, alkaloid, and tannin
which have the function to improve the repair and
strengthen the skin cells and stimulate the growth of
connective tissue. Krishnaiah et al. (2009) said that
triterpenoids are components that have an active role
in wound healing. Triterpenoid help strengthens the
skin structure, increase the concentration of
antioxidants and restore inflammatory or inflamed
tissue by increasing blood supply to the wound area
and accelerate the process of wound healing.
Loquat leaves have secondary metabolite
compounds such as triterpenes, flavonoids, tannins,
which have an affect as an antiinflammatory,
antiviral, antioxidant (Banno et al., 2005),
antibacteria, antiallergic (Tan et al., 2017).
Triterpene compounds in Centella asiatica, mainly
including two glycosides are considered to facilitate
burn wound healing via activating growth factors
such as TGF-β that favors fibroblast proliferation
and could elevate collagen synthesis. The
compounds can accelerate the time span of the
wound healing process (Wu et al., 2012).
The hemostasis/inflammation phase of acute
wound healing reflects the time recruit the many
celullar elements in several days to weeks that are
activated during early repair and the absence of
mechanical strength (Robson et al. 2001). So that, PI
has a potential effect on wound healing to the
average time span of wound healing.
Macroscopical examination of wound area of
each group (Mus musculus L.) in the day 1
st
, day 7
th
,
day 14
th
can be seen in Figure 1.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
164
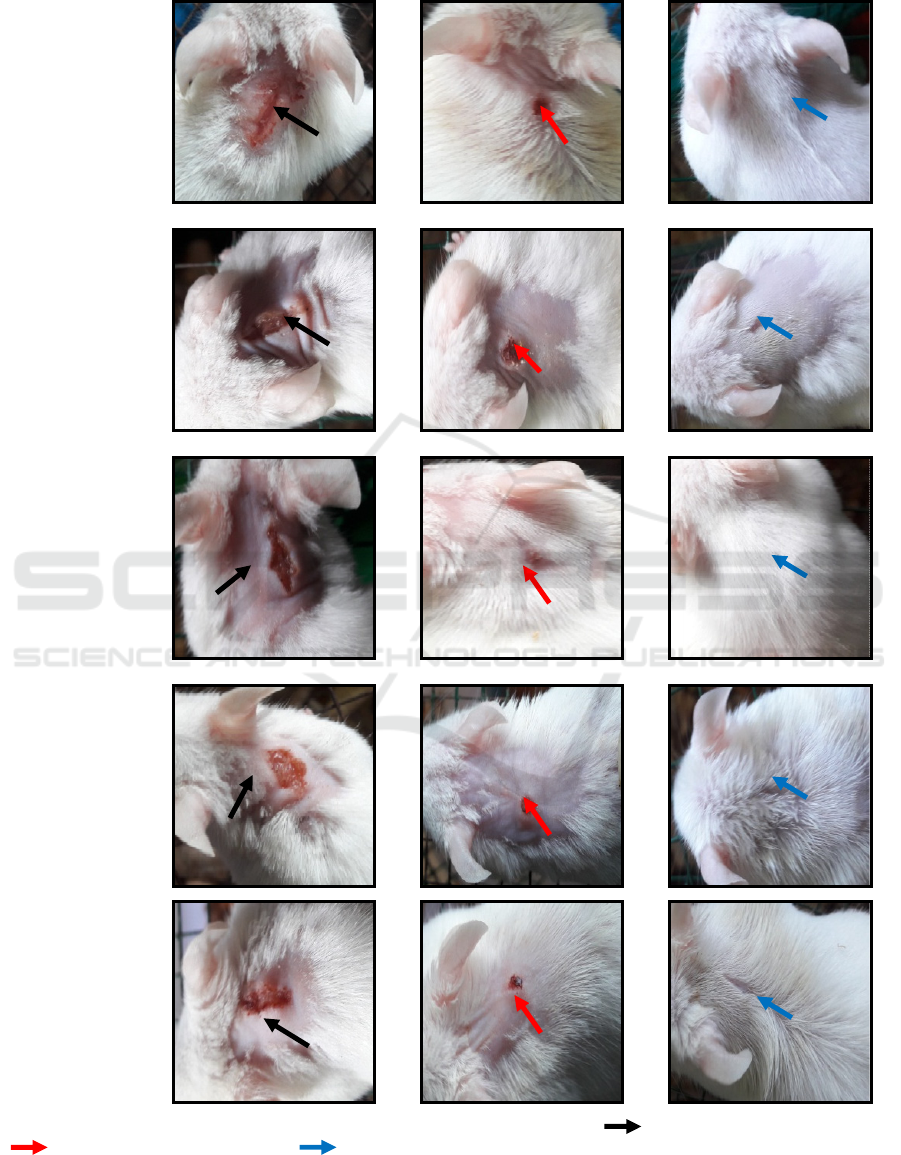
Groups
Day 1
st
Day 7
th
Day 14
th
K(-)
(Negative
control)
K+
(Positive
control)
PI (loquat leaves
extract with
concentration
15%
PII (loquat
leaves extract
with
concentration
30%)
PIII (loquat
leaves extract
with
concentration
45%)
Figure 1 : macroscopical examination of wound area of each group of treatment.( ) day 1
st
, wounds red and swollen,
( ) day 7
th
, wounds shrink and dry, ( ) day 14
th
, wounds healed.
Effectivity Test of Loquat (Eriobotrya japonica (Thunb.) Lindl.) Leaves Extract on the Incision Wound Healing in Mice (Mus musculus L.)
165

3.2 The Average Number of Fibroblast
Cells
Histological observations of the fibroblast cells on
the histological preparations of the mice skin with a
magnification of 400 x. Data on the average number
of fibroblast cells in each group on the day 14
th
can
be seen in Table 3.
Table 3: The average number of fibroblast cells.
Grou
p
s Fibroblasts ± SD
K
(
-
)
6,42
a
± 4,36
K(+) 12,26
b
± 2,93
PI 19,16
c
± 5,83
PII 15,42
bc
± 3,85
PIII 14,74
bc
± 3,55
Based on Table 3, it can be seen that the highest
average number of fibroblasts on the day 14
th
was in
the treatment group, a concentration of 15% (PI)
with 19.16, while the lowest was in the negative
control treatment group (K-) with 6.42.
Based on the statistical test, data was obtained
significant results 0.002 (p <0.05). Followed by the
Duncan test and the result is that the treatment group
concentration 15% (PI) is the group that the most
potential on wound healing seen from the number of
fibroblasts.
The effect of ethanol extract of loquat leaves on
the number of fibroblasts is shown from the lower
extract concentration, the higher the number of
fibroblasts. The use of ethanol extracts of loquat
leaves might effect the addition of nutrients derived
from loquat leaves in the wound area which can
optimize wound healing by increasing the number of
fibroblasts.
Alkaloid compound can accelerate soft tissue
repair. Reyes et al., (1993) presented evidence that
alkaloid compound, taspine, extracted from Croton
lechleri by its chemotactic process toward
fibroblasts that migrate into the wound from local
tissues and increasing extracellular matrix synthesis
due to their increased number.
Two glycoside triterpene compounds were able
to enhance collagen type I and type III synthesis
mainly through activating skin fibroblasts (Wu et al.,
2012). Collagen fibers type III is synthesized first in
the proliferation phase by fibroblasts that are
stimulated by growth factors TGF-β from fibroblast
cells and macrophages itself (Sabirin et al., 2013).
The large amount of connective tissue in the
wound area could help accelerate the wound
contraction. So, the wound side will be contracted
and caused the wound area to be smaller (Prasetyo et
al., 2010).
Fibroblasts synthesize and release
glycosaminoglycans and proteoglycans, which are
also important components of the extracelullar
matrix of granulation tissue. Simultaneously,
vascular generation (angiogenesis) occurs with the
use of the maturing matrix. The acute wound
fibroblast density reaches a maximum between 7 and
14 days after injury (Robson et al., 2001).
Fibroblast is one indicator that healing process
occurred faster. Increasing of fibroblast as a result of
lymphokines induced which secreted by CD4
+
lymphocytes, which has a role as healing promotor
to cellular immune response. Depletion of CD4
+
lymphocytes can decrease skin tension, angiogenesis
and extracellular matrix component (Prasettyono,
2009).
3.3 The Average Number of
Lymphocyte Cells
Histological observations of the lymphocyte cells on
the histological preparations of the mice skin with a
magnification of 400 x. Data on the average number
of lymphocyte cells in each group on the day 14
th
can be seen in Table 4.
Table 4: The average number of lymphocyte cells.
Grou
p
sL
y
m
p
hoc
y
te ± SD
K
(
-
)
5,10
a
± 2,96
K(+) 4,70
a
± 2,04
PI 18,08
c
± 3,97
PII 11,22
b
± 3,63
PIII 13,38
b
± 4,98
Based on Table 4, it can be seen that the highest
average number of lymphocytes on the day 14
th
was
in the treatment group, a concentration of 15% (PI)
with 18.08, while the lowest was in the positive
control treatment group (K+) with 4.70.
Based on the statistical test, data was obtained
significant results 0.000 (p <0.05). Followed by the
Duncan test and the result is that the treatment group
concentration 15% (PI) is the group that the most
potential on wound healing seen from the number of
lymphocytes.
The effect of ethanol extract of loquat leaves on
the number of lymphocytes is shown from the lower
extract concentration, the higher the number of
lymphocytes. Secondary metabolite compounds in
loquat leaves might able to accelerate the
inflammatory phase as seen from the high number of
lymphocytes in the treatment group of loquat leaves
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
166
extract which can inhibit the growth and kill
microorganisms in the wound area.
Loquat fruit and leaves have high concentration
of Vitamin A. Vitamin A can play a role in
accelerating the inflammatory phase to the
proliferation phase by increasing monocytes,
lymphocytes and macrophages to the wound area
which will eliminate bacteria from the wound area
and produce growth factors needed for proliferation
of fibroblast cells and angiogenesis (Kumar et al.,
2014; Negara et al., 2014).
Lymphocytes were present within the wound at
one day, increased to peak numbers between days 8
and 14 post-wounding and remained present.
Lymphocytes are an important regulator of
fibroblast activity both directly and indirectly
through the macrophages during wound healing.
Martin and Muir, 1990).
On the day 7th, inflammatory cells and
macrophages begin to migrate together with
fibroblast cells into the wound tissue. Fibroblasts
will proliferate with the help of growth factors,
especially transforming growth factor -β (TGF-β)
and basic fibroblast growth factor (bFGF) which are
secreted by platelets and macrophages. Macrophages
will experience a reduction in the number as a result
of tissue repair which process will be followed by
fibroblast, endothelial cells and (Sabirin et al.,
2013
).
On proliferation phase, CD4
+
lymphocytes
induce keratinocytes to release IL-1 in the wound
area. Keratinocytes have a potential role on
epithelization, proliferation, and maturation of
epidermis. IL-1 that has been released by
keratinocytes induces endothelial cells to form
angiogenesis and fibroblast to form extracellular
matrix (Prakoso and Kurniasih, 2018).
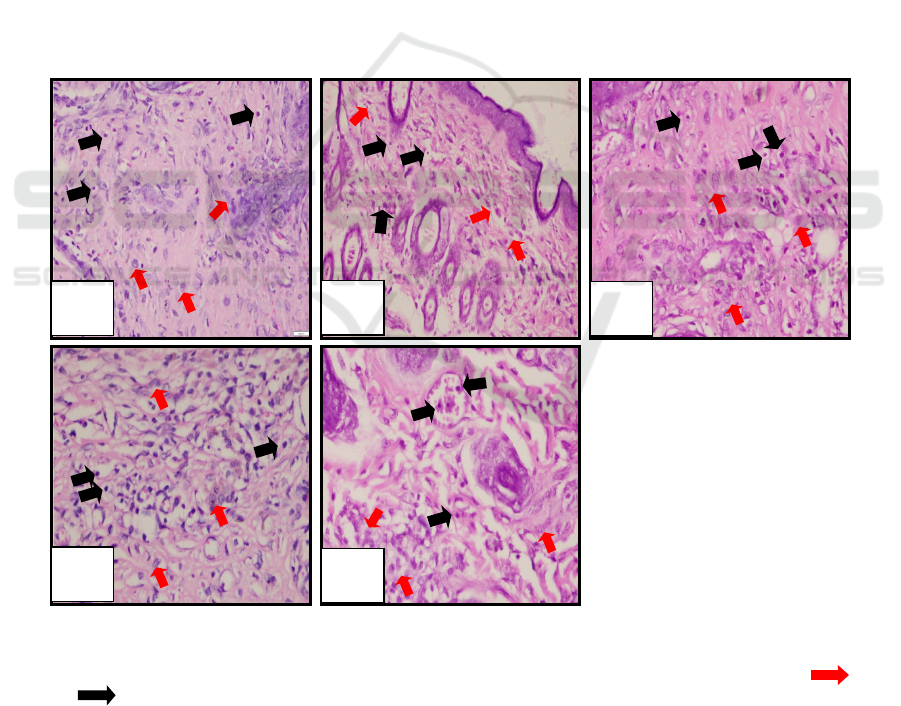
The histological figure of fibroblast and
lymphocytes of mice skin on day 14
th
with 400x
magnification can be seen in Figure 2 below:
Figure 2: Microscopical examination of fibroblasts and lymphocytes.
(a) negative control (K-); (b) positive control (K+); (c) loquat leaves extract with concentration 15% (PI);
(d) loquat leaves extract with concentration 30% (PII); (e) loquat leaves extract with concentration 45% (PIII), ( )
fibroblas ( ) limfosit.
According to Balqis et al., (2014) that on the 14
th
day of the histological slide, infiltration of
inflammation cells was still visible and collagen
fibers have spread. In normal tissue, fibroblast cells
are rarely found. After the injury, fibroblast will
actively migrate to the wound area, will proliferate
collagen which plays a role in new tissue formation
until the skin returns to normal.
a
b
c
d
e
Effectivity Test of Loquat (Eriobotrya japonica (Thunb.) Lindl.) Leaves Extract on the Incision Wound Healing in Mice (Mus musculus L.)
167
3.4 The Average Number of Epithelial
Thickness
Histological observations of the epithelial thickness
on the histological preparations of the mice skin
with a magnification of 100x. Observation of
epithelial thickness is measured from the stratum
corneum layer to the stratum basale. Data on the
average epithelial thickness in each group on the
day 14
th
can be seen in Table 5.
Table 5: The average number of epithelial thickness.
Groups Ephitelial thickness (µm) ± SD
K
- 88,18
a
± 27,71
K+ 110,60
ab
± 41,99
PI 171,61
c
± 35,62
PII 166,12
c
± 40,85
PIII 156,88
bc
± 48,59
Based on Table 5, it can be seen that the highest
average number of epithelial thickness on the day
14
th
was in the treatment group, a concentration of
15% (PI) with 171,61 µm, while the lowest was in
the positive control treatment group (K+) with 88,18
µm.
Based on the statistical test, data was obtained
significant result 0.010 (p <0.05). Followed by the
Duncan test and the result is that the treatment group
concentration 15% (PI) is the group that the most
potential on wound healing seen from the number of
epithelial thickness.
Group PI, PII and PIII has shown the highest
number. The epithelial thickness can indicate the
faster process of reepithelization, so that can
accelerate the wound healing. The faster process in
the treatment group might be affected by loquat
leaves extract. Loquat leaves contain tannin, vitamin
A, Pratiwi et al. (2015) suggested that vitamin A, C,
E, tannins. and saponins in clove flower bud extract
can help the process of reepithelization by increasing
the differentiation of epithelial cells.
Loquat contain secondary metabolites such as
alkaloids which have antibacterial and antioxidant
effects that are high enough to maintain skin
integrity (Kumar et al., 2014), and enough to play a
role in the wound healing process by increasing
collagen formation, differentiation of epithelial cells
and increasing immunity (Negara et al., 2014). The
antioxidant effect can thicken the epithelial layer.
Substitution epithelial tissue occurs on the surface of
epithelial cells that continue to experience cell death
(Yohana, 2015).
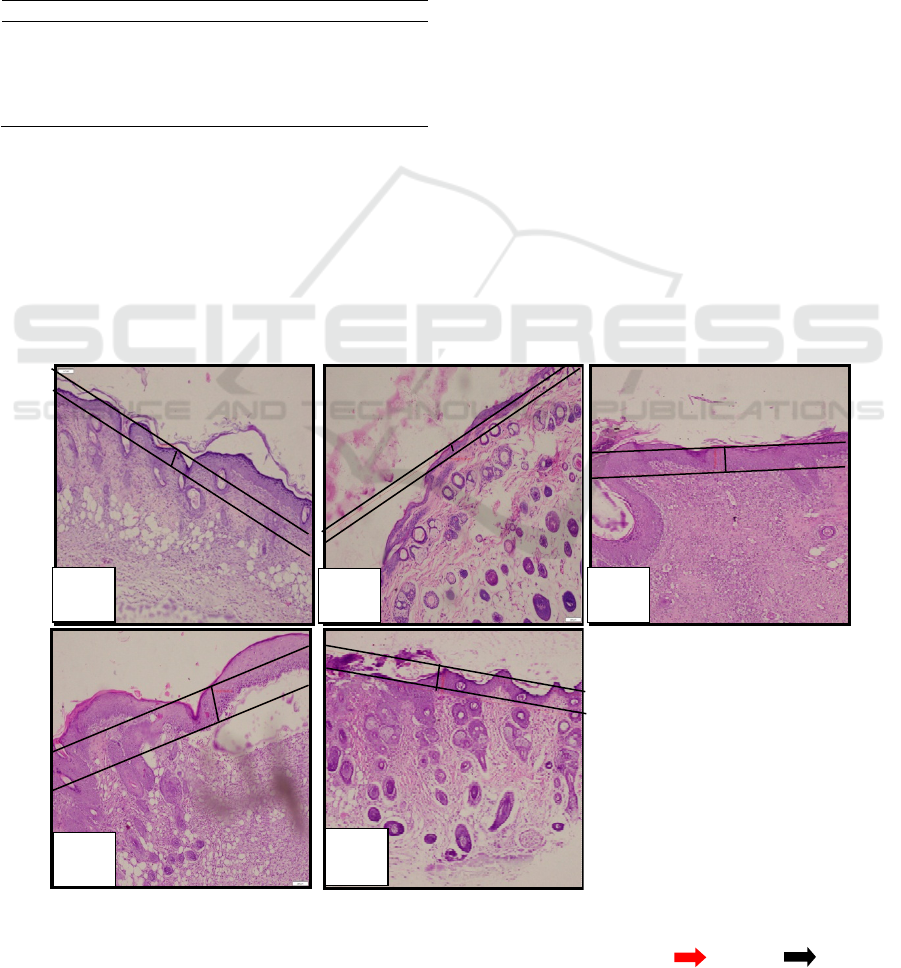
Microscopical examination of epithelial
thickness with 100x magnification can be seen in
Figure 3.
Figure 3: Microscopical examination of ephitelial thickness.
(a) negative control (K-); (b) positive control (K+); (c) loquat leaves extract with concentration 15% (PI); (d) loquat leaves
extract with concentration 30% (PII); (e) loquat leaves extract with concentration 45% (PIII), ( )fibroblas ( ) limfosit.
a
b
c
d
e
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
168

4 CONCLUSIONS
The average time span of wound healing for each
group K-, K +, PI, PII, and PIII were subsequently 8
days, 9.2 days, 7.6 days, 8.4 days, and 9.4 days.
Wounds treated with 15% concentration of loquat
leaf ethanol extract (PI) showed the fastest healing
effect while the 45% concentration of loquat leaf
ethanol extract (PIII) showed the longest healing
effect. The average number of epithelial thickness,
fibroblasts, and lymphocytes in the treatment group
of loquat leaf ethanol extract with a concentration
15% (PI) is higher than the other treatment groups
with the significant results of statistical analysis.
REFERENCES
Balqis, U., Masyitha, D., Febrina, F., 2014. Proses
Penyembuhan Luka Bakar dengan Gerusan Daun
Kedondong (Spondias dulcis F.) dan Vaselin pada
Tikus (Rattus novergicus) secara Histopatologis.
Jurnal Medika Veterinaria. 8 (1): 11-12.
Banno, N., Akihisa, T., Tokuda, H., Yasukawa, K.,
Taguchi, Y., Akazawa, H., Ukiya, M., Kimura, Y.,
Suzuki, T., Nishino, H., 2005. Anti-inflammatory and
Antitumor-Promoting Effects of the Triterpene Acids
from the Leaves of Eriobotrya japonica. Biological
and Pharmaceutical Bulletin. 28 (10): 1995.
Kimura, Y., Sumiyoshi, M., Samukawa, K., Satake, N.,
Sakanaka, M., 2008. Facilitating Action of
Asiaticoside at Low Doses on Burn Wound Repair and
its Mechanism. European Journal of Pharmacology.
584: 414-423.
Krishnaiah, D., Devi, T., Bono, A., Sarbatly, R., 2009.
Studies on Phytochemical Constituents of Six
Malaysian Medicinal Plants. Journal of Medicinal
Plants Research. 3 (2): 070.
Kumar, S., Ritu., Pallavi, G., 2014. A Critical Review on
Loquat (Eriobotrya japonica Thunb/Lindl.).
International Journal of Pharmaceutical and
Biological Archives. 5(2): 1-7.
Kusumawardhani, AD., Kalsum, U., Rini, IK., 2015.
Pengaruh Sediaan Salep Ekstrak Daun Sirih (Piper
betle Linn.) terhadap Jumlah Fibroblas Luka Bakar
Derajat IIA pada Tikus Putih (Rattus novergicus L.)
Galur Wistar. Majalah Kesehatan FKUB. 2 (1): 19,
20.
Lee, J., Jung, E., Kim, Y., Park, Y., Park, J., Hong, S.,
Kim, S., Hyun, C., Kim, S., Park, D., 2006.
Asiaticoside Induces Human Collagen I Synthesis
through TGFβ Receptor I Kinase (TβRI Kinase)-
Independent Smad Signaling. Planta Med. 72: 324-
328.
Liu, M., Dai, Y., Li, Y., Luo, Y., Huang, F., Gong, Z.,
Meng, Q., 2008. Madecassoide Isolated from Centella
asiatica Herbs Facilitates Burn Wound Healing in
Mice. Planta Med. 74: 809-815.
Martin, CW., Muir, IFK., 1990. The Role of Lymphocytes
in Wound Healing. British Journal of Plastic Surgery.
43: 659-660.
Negara, RFK., Ratnawati, R., SLI, DD., 2014. Pengaruh
Perawatan Luka Bakar Derajat II Menggunakan
Ekstrak Etanol Daun Sirih (Piper Betle Linn.) terhadap
Peningkatan Ketebalan Jaringan Granulasi pada Tikus
Putih (Rattus novergicus) Jantan Galur Wistar.
Majalah Kesehatan FKUB. 1(2) : 92.
Nurwahyuni, I., Marpaung, HN., Rahayu, S., 2017. In
Vitro Germination of Anti-Diabetic Plant Loquat
(Eriobotrya japonica Lindl.) to Produce Good
Seedling. Biotechnology. 8 (4): 31.
Prasettyono, TOH., 2009. General Concept of Wound
Healing. Revisited. Med J Indones. 18 (3): 209-210.
Prasetyo, BF., Wientarsih, I., Priosoeryanto, BP., 2010.
Aktivitas Sediaan Gel Ekstrak Batang Pohon Pisang
Ambon dalam Proses Penyembuhan Luka Pada
Mencit. Jurnal Veteriner. 11 (2): 71.
Pratiwi, AD., Ratnawati, R., Kristianto, H., 2015.
Pengaruh Pemberian Ekstrak Kuncup Bunga Cengkeh
(Syzygium aromaticum) terhadap Peningkatan
Ketebalan Epitelisasi Luka Insis pada Tikus Putih
(Rattus Novergicus) Galur Wistar. Majalah Kesehatan
FKUB. 2 (3): 140-141.
Perdanakusuma, DS., 2007. Anatomi Fisiologi Kulit dan
Penyembuhan Luka. Airlangga University School of
Medicine. Surabaya.
Prakoso, YA., Kurniasih., 2018. The Effects of Aloe vera
Cream on the Expression of CD4
+
and CD8
+
Lymphocytes in Skin Wound Healing. Journal of
Tropical Medicine. 2018. 3-5.
Rahman, S., Kosman, R., Mukrima, I., 2013. Efek Ekstrak
Etanol Daun Awar-Awar (Ficus septica Burm. F)
Terhadap Kemampuan Epitelisasi Pada Tikus (Rattus
novergicus). Jurnal Bionature. 14 (2): 114-116.
Reyes, BHP., Lewis, W., Roman, J., Simchowitz, L.,
Mustoe, TA., 1993. Enhancement of Wound Healing
by the Alkaloid Taspine Defining Mechanism of
Action. Experimental Biology and Medicine. 203 (18)
: 21-23.
Robson, MC., Steed, DL., Franz, MG., 2001. Wound
Healing: Biologic Features and Approaches to
Maximize Healing Trajectories. Current Problems in
Surgery. 38 (2): 78, 79, 97.
Ross, MH., Pawlina, W., 2011. Histology. A Text and
Atlas. Lippincott Williams and Wilkins. Philadelphia.
Sabirin, IPR., Maskoen, AM., Hernowo, BS., 2013. Peran
Ekstrak Etanol Topikal Daun Mengkudu (Morinda
citrifolia L.) pada Penyembuhan Luka. Majalah
Kedokteran Bandung. 45 (4) : 228-232.
Suntoro, SH., 1983. Metode Pewarnaan. Histologi dan
Histokimia. Penerbit Bhratara Karya Aksara. Jakarta.
Szycher, M., Lee, SJ., 1992. Modern Wound Dressings : A
Systematics Approach to Wound Healing. Journal of
Biomaterials Applications. 7 (142): 148.
Tan, H., Sonam, T., Shimizu, K., 2017. The Potential of
Terpenoids from Loquat Leaves (Eriobotrya japonica
Effectivity Test of Loquat (Eriobotrya japonica (Thunb.) Lindl.) Leaves Extract on the Incision Wound Healing in Mice (Mus musculus L.)
169

(Thunb.) Lindl.) for Prevention and Treatment of Skin
Disorder. International Journal of Molecular Sciences.
18 (1030): 2.
Thakur R, Jain N, Pathak R, Sandhu S, 2011. Practices in
Wound Healing Studies of Plants. Evidence-Based
Complementary and Alternative Medicine. 2011: 8.
Velnar, T., Bailey, T., Srkolj, V., 2009. The Wound
Healing Process: an Overview of the Cellular and
Molecular Mechanisms. The Journal of International
Medical Research. 37 (5): 1528.
Wu, F., Bian, D., Xia, Y., Gong, Z., Tan, Q., Chen, J., Dai,
Y., 2012. Identification of major Active Ingredients
Responsible for Burn Wound Healing of Centella
asiatica Herbs. Evidence Based Complementary and
Alternative Medicine. 2012. 1-12.
Yohana, W., Suciati, A., Rachmawati, M., 2015.
Peningkatan Ketebalan Epitel Mukosa Bukal setelah
Aplikasi Ekstrak Daun Sirih. Majalah Kedokteran
Gigi. 1(1). 25.
Zhang, J., Li, Y., Chen, S., Zhang, L., Wang, J., Yang, Y.,
Zhang, S., Pan, Y., Wang, Y., Yang, L., 2015.
Systems Pharmacology Dissection of the Anti-
Inflammatory Mechanism for the Medicinal Herb
Folium Eriobotryae. International Journal of
Molecular Sciences. 16 (2015): 2915, 2919.
Zheng, C., Qin, L., 2007. Chemical Components of
Centella asiatica and Their Bioactivities. Journal of
Chinese Integrative Medicine. 5 (3): 849.
[Kemenkes] Kementerian Kesehatan RI, Direktorat
Jenderal Bina Kefarmasian dan Alat Kesehatan, 2013.
Suplemen III Farmakope Herbal Indonesia. Edisi 1.
Kementerian Kesehatan RI. Jakarta.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
170
