
Carboxymethyl Starch Synthesis from Breadfruit Starch (Artocarpus
Communis) through Esterification Reaction with Monochloro Acetate
Cut Fatimah Zuhra
1*
, Mimpin Ginting
1
, Arny Masyita
1
and Wilza Fithri Az-zahra
1
1
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan, Indonesia
Keywords: Breadfruit Flesh, Carboxymethyl Starch (CMS), Monokloroacetate, Starch.
Abstract: The synthesis of carboxymethyl starch from breadfruit starch (Artocarpuscommunis) has been done through
the etherification reaction with monochloroacetate. The first stage is the isolation of starch from the fruits of
breadfruit fruits, where the results of FTIR analyzed obtained spectra with 3402, 2931, 1080, 1018 cm-1
waves indicating the presence of O-H, C-H stretching and C-O-C bonds, illustrating that the compound is a
starch compound. The second stage is the etherification process with monochloroacetate using NaOH
reagent and isopropanol as solvent. The carboxymethylation process using monochloroacetate was carried
out with variations in the addition of monochloroacetate 1.5; 3; 4.5; 6; 7.5 grams and 90 times variations;
120; 150 minutes and neutralization using 2N CH
3
COOH. The resultant carboxymethyl starch shows the
appearance of peaks at the wavenumbers 1604 cm-1 and 1419 cm-1 in the analysis using FTIR spectroscopy
showing the presence of carbonyl groups. Then the carboxymethyl starch produced was calculated by
degrees of substitution and SEM analysis was performed. The highest degree of substitution was 1.8412 in
weight gain of 6 grams of monochloroacetic acid with reaction time of 2 hours and obtained a rougher
surface shape due to the presence of granules and a more unified appearance.
1 INTRODUCTION
Breadfruit (Artocarpus communis) has the potential
as a national food security reserve because breadfruit
can produce throughout the year. Besides, breadfruit
contains nutrients that are not inferior to corn or
tubers. This plant has long been cultivated by the
people of Indonesia, but for the people of Indonesia
consumption of breadfruit is generally still limited as
a snack and vegetable (Pitojo, 1992). As one
alternative food source, breadfruit is proven to have
high nutritional content (Widowati, 2003).
Breadfruit has high carbohydrate content because it
is a valuable source of starch. Starch obtained from
breadfruit produces 18.5 g / 100 g with a purity of
98.86% with an amylose content of 27.68% and
amylopectin 72.32% (Rincom, A.M. & Fanny,
2004).
The use of starch in the industry is very broad,
both in the field of food and non-food because of the
ease of getting raw materials and the price is
relatively cheap. However, some properties of
natural starch become an obstacle if used as
industrial raw materials, including the nature of
starch which is easily damaged by heat and acid
(Sangseethong et al., 2005). The commonly used
way to overcome these weaknesses is to change the
molecular structure of starch physically, chemically
or combine which will improve the properties of
natural starch (Liu, 2005).
Modification of starch is done by cutting the
molecular structure, rearranging the molecular
structure through oxidation or substitution of
functional groups in starch molecules (Wurzburg,
1989). Carboxymethylation is a modification
method by substituting the starch molecular function
groups. This modification produces starch with low
gelatinization temperature, high solubility and high
shelf life (Sangseethong et al., 2005). The
carboxymethylation process takes place by
substituting a natural starch hydroxyl group (-OH)
with a carboxymethyl group (-CH
2
COO-) to produce
Na-carboxymethyl starch or carboxymethyl starch
(CMS) (Sangseethong et al., 2005). Utilization of
Na-carboxymethyl starch, among others, as a
disintegrant in the pharmaceutical industry (Shah &
Augsburger, 2002) and as a sizing and printing agent
in the textile industry (Ragheb et al., n.d.).
(Fachrudin, 2013) has researched Na-
Carboxymethyl starch production with different
Fatimah Zuhra, C., Ginting, M., Masyita, A. and Fithri Az-zahra, W.
Carboxymethyl Starch Synthesis from Breadfruit Starch (Artocarpus Communis) through Esterification Reaction with Monochloro Acetate.
DOI: 10.5220/0010137800002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 143-148
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
143

types of stirring at the alkalization stage, the results
obtained show that the greater the concentration of
NaOH and on the type of stirring carried out with
homogenizer will produce higher substitution
degrees. Synthesis and antibacterial of
carboxymethyl starch poly-branch (vinyl imidazole)
against some plant pathogens produce a degree of
substitution of 0.81 and rupture of starch granules
under the influence of alcohol, alkali and heat
environment which replaces OH groups with
carboxymethyl groups and afterwards N-vinyl
imidazole is grafted to CMS in water using
potassium persulfate as an initiator at 450C (El-
Hamshary et al., 2014).
From the background described above,
researchers are interested in examining the synthesis
of carboxymethyl starch through the reaction of
breadfruit starch etherification with variations in the
addition of Monochloro Acetate and reaction time.
2 MATERIALS AND METHODS
2.1 Materials
The materials used in this study include: Breadfruit,
Aquadest, Isopropanol, NaOH, Monochloroacetic
Acid, Acetic Acid, Acetone, HCl, Ethanol, PP
indicators.
2.2 Methods
2.2.1 Isolation of Starch from Breadfruit
Breadfruit that is old or whose skin begins to turn
yellow is peeled and the fruit stalk is removed. After
peeling, the breadfruit is washed until it is free of
dirt and sap. Then breadfruit cut into small pieces,
then mashed using a blender. Breadfruit that has
been mashed is filtered using gauze, the filtrate from
the filter is left to form a precipitate. The precipitate
obtained is washed repeatedly with water until the
upper layers are clear. The starch obtained was dried
in an oven at 45°C for 24 hours. The dried starch is
then mashed, sieved, weighed and stored in a
desiccator. Subsequently analyzed by IR
spectroscopy.
2.2.2 Preparation of Carboxymethyl Starch
from Pati Breadfruit
Add 5 grams of starch and 210 mL of isopropanol
and 30 mL Aqudest into a three-neck flask, then the
mixture is stirred with a magnetic stirrer at a speed
of 400 rpm for 4 hours at a temperature of 35°C.
Then 1.6 grams of NaOH has been dissolved with 25
mL aqua dest while stirred for 45 minutes and the
temperature raised to 45°C. Then added 1.5 grams of
Monochloro acetate which has been dissolved in
12.5 mL isopropanol and mixed with 1.5 grams of
NaOH dissolved in 12.5 mL Aquadest and stirred for
2 hours. Then cooled and then the mixture was
neutralized with 2 N. acetic acid. Then added
acetone and filtered. The precipitate was washed
with acetone-water (60:40 v/v) and pure acetone.
And the end product carboxymethyl starch is dried
in an oven 50°C.
The procedure was carried out with the variation
in weight of Monochloro acetate 1.5; 3; 4.5; 6; 7.5
grams and reaction time of 1.2; 2; 2.5 hours.
Carboxymethyl starch obtained was analyzed by FT-
IR and SEM, the degree of substitution was
calculated.
2.2.3 Determination of Degree of
Substitution (ISO 11216-1998)
Carboxymethyl starch samples were converted to
acidic form with the addition of HCl. A sample of
1.5 g was added with 45 ml of acetone, added with
3.75 ml of HCl 6M and stirred with a stirrer for 30
minutes. The filtered solution is then dispersed in
80% ethanol, filtering the solution to neutral pH.
The filtration results are dispersed in absolute
ethanol, then filtered and dried for 24 hours in an
oven at 50°C. 0.5 g of sample was dissolved in 25
ml of 0.1 M NaOH and added with 75 ml of distilled
water. Samples were then titrated using 0.1 M HCl
with phenolphthalein indicator (ISO 11216-1998).
2.2.4 Functional Group Analysis with FT-IR
Spectroscopy
Carboxymethyl starch was mashed with pestle and
mortar and then made into pellets with KBr and
spectra were measured with FT-IR spectroscopy.
2.3.5 SEM Analysis (Scanning Electron
Microscopy)
The sample is placed in a cell holder with a double
lid. The sample is inserted into a Scanning Electron
Microscopy (SEM), then the surface image is
observed and magnified as desired. Then a
photoshoot is taken.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
144
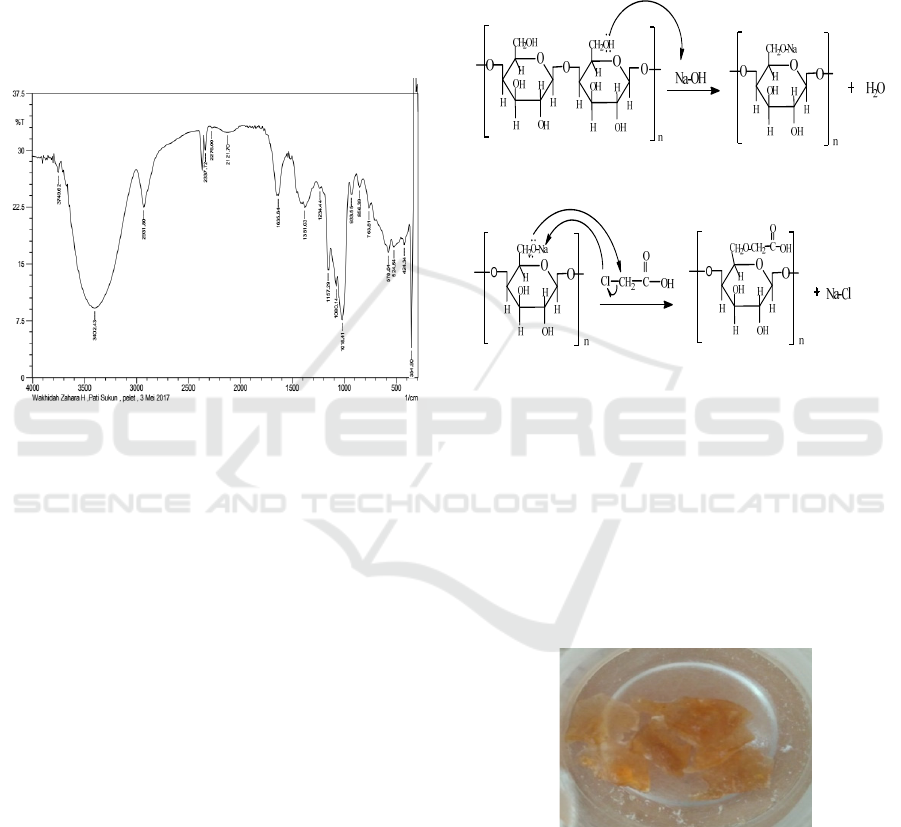
3 RESULTS AND DISCUSSION
3.1 FTIR Spectrophotometer Analysis
of Breadfruit Starch
Isolated starch from 8 breadfruits with a mass of
about 18 kg is 470 grams (2.61%). FT-IR
spectroscopy data of breadfruit starch provide
spectrum with vibrational peaks in the region of
wave numbers 3402, 2931, 2121, 1381, 1080, and
1018 cm-1 (Figure 1).
Figure 1: FTIR spectroscopy of breadfruit starch.
The spectrum shown from FT-IR data gives
support that the starch obtained has an OH group
with the emergence of a vibration peak at wave
number 3402 cm-1, supported by the emergence of a
stretching CH group at number 2931 cm-1, and the
carbonyl group (C = O) at 1635 cm-1 (Figure 1)
corresponding to commercial starch (Nurafrida,
2011) and COC bonds shown at numbers 1080 and
1018 cm-1 (Ochoa, 2013).
3.2 Carboxymethyl Starch
Carboxymethyl starch is produced through the
process of etherification with Monochloro acetate
(MCA) in an alkaline atmosphere. The first stage is
alkalization with NaOH as a promoter and producing
alkaline starch. Starch before entering the
alkalization stage is dispersed first in an isopropanol
solvent. Isopropanol functions as a reaction medium,
besides that isopropanol, will also dissolve minor
components such as fiber, ash, fat, and protein. The
dispersed starch then undergoes a stirring process.
The alkalization stage is the opening step to
activate the starch hydroxyl group (St-OH) into a
negatively charged alkoxide group (St-O-). The
alkalization stage creates a stress-strain on adjacent
starch molecules, this will weaken the starch double
helix bond area and damage the starch crystalline
structure (Chen & Jane, 1994). This condition will
facilitate the solvent and MCA to enter the starch
granules and substitute the alkoxide group with the
carboxymethyl group from the MCA (Kooijman et
al., 2003).
Figure 2: The reaction mechanism of an experiment
making carboxymethyl starch.
The variation made in this study is the weight
variation of Monokoloroetetat which is 1.5, 3, 4.5, 6,
and 7.5 grams and the reaction time is 90, 120 and
150 minutes. The carboxymethyl starch obtained in
the form of a carboxymethyl solid can theoretically
be seen in Figure 3. The light brown color is
transparent, where the carboxymethyl starch
resulting from synthesis is 4,50 g respectively; 4.82
g; 4.77g; 4.95 g; 4.65 g; and 4.71 g; 4.95 g; 4.54g.
Figure 3: Synthesis of carboxymethyl starch.
3.3 FTIR Spectrophotometer Analysis
of Carboxymethyl Starch
Carboxymethyl starch was analyzed using FT-IR
spectroscopy. FT-IR spectrum results using 1.5 gram
Monocloroacetate; monochloroacetate 3g; 4.5g
monochloroacetate; 6g monochloroacetate;
Carboxymethyl Starch Synthesis from Breadfruit Starch (Artocarpus Communis) through Esterification Reaction with Monochloro Acetate
145
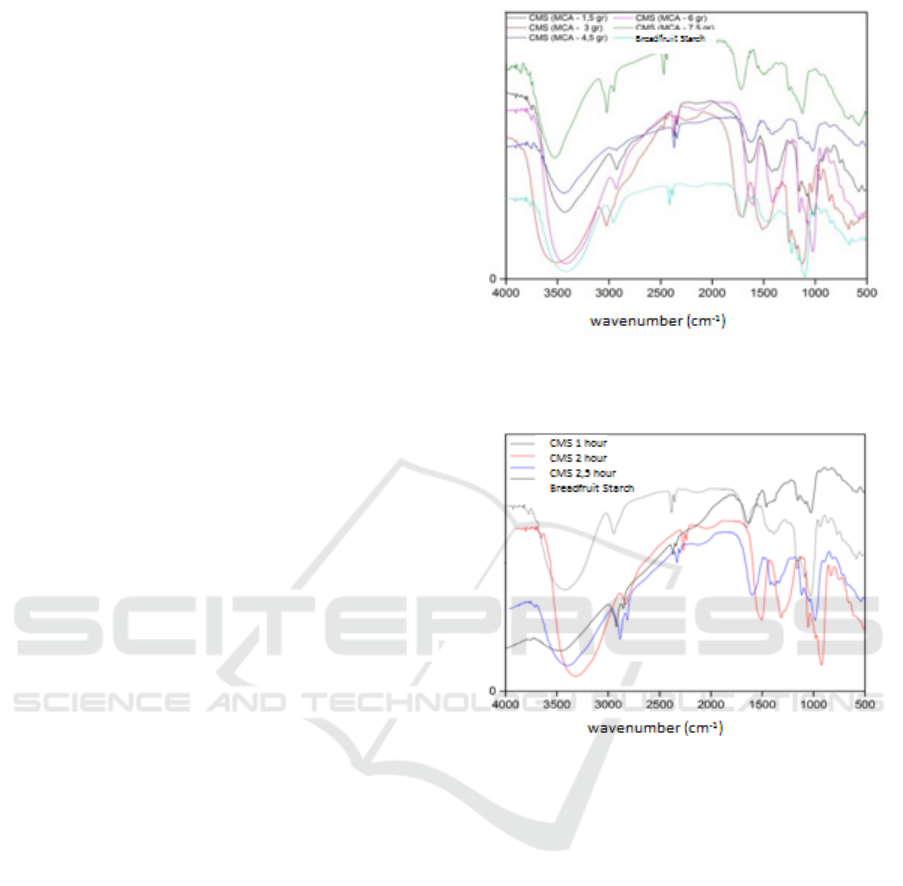
Monochloroacetate 7.5g and with an etherification
reaction time of 90 minutes; etherification reaction
time of 120 minutes; the etherification reaction time
of 150 minutes has shown vibrations in the region of
wave numbers 3417, 2931, 1604, 1419, 1373, and
1026 cm
-1
.
The formation of carboxymethyl starch was
shown in the results of FT-IR analysis of
monochloroacetate weight variation and reaction
time. Characterized by the emergence of vibration
peaks in the region of wave numbers 1601-1408
cm
-1
which shows the COO region (El-Hamshary et
al., 2014). It was also stated by (Zhang, 2012) and
(Ochoa, 2013) that the emergence of the COO group
was marked by the peak spectra of 1618 cm
-1
and
1424 cm
-1
as well as 1605 cm
-1
and 1417 cm
-1
. In
starch carboxymethyl, there was a change in the
intensity of the carbonyl group in the 1604 cm
-1
and
1419 cm
-1
regions. Changes in the intensity of starch
carboxymethyl carbonyl groups with variations in
the weight of monochloroacetate 1.5; 3; 4.5; 6; and
7.5 grams with a 120 minute etherification reaction
time of 1635 cm
-1
and 1419 cm
-1
; 1604 cm
-1
and
1411 cm
-1
; 1620 cm
-1
and 1419 cm
-1
; 1604 cm
-1
and
1419 cm
-1
; 1620 cm
-1
and 1458 cm
-1
. From the FT-
IR data, the highest intensity of the carbonyl group
is at 6 grams of monochloroacetate weight. Starch
weighing 6 grams of monochloroacetate was
continued with time variations of 90 and 150
minutes, and the intensity of the carbonyl group was
1635 cm
-1
and 1458 cm
-1
; 1635 cm
-1
and 1458 cm
-1
.
This shows the occurrence of the addition of
carboxylic groups to starch carboxymethyl. From
this data, the most optimum is carboxymethyl starch
with 6 gram Monocloroacetate weight with 120
minutes reaction time, because the highest intensity
of the carboxylate group is 11,072. Comparison of
the FT-IR spectrum of monochloroacetic weight
variation and reaction time variation can be seen in
Figure 4 and Figure 5.
Figure 4: FTIR spectrum of carboxymethyl starch by
monochloroacetate weight variation.
Figure 5: FTIR spectrum of carboxymethylstarch variation
in reaction time.
3.4 Determination of Substitution
Degree
Determination of the degree of substitution titration
method based on ISO 11216-1998. In the weight
variation of monocloroacetate, the highest
carboxylate content was obtained, with 6 grams of
monocloroacetate treatment at 1.8412 per AGU. The
results of determining the degree of substitution with
variations in weight Monocloroacetate and
variations in the time of the etherification reaction as
in Table 1 and Table 2.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
146

Table 1: Degrees of starch carboxymethyl substitution
with variation in weight Monochloroacetate.
Weight
(
C
8
H
12
O
8
)
n
(g)
Degree of Substitution
(
by
AGU
)
1.5
3
4.5
6
7.5
0.1669
0.5823
1.1036
1.8412
0.8160
Table 2: Degrees of starch carboxymethyl substitution
with variations in reaction time
.
Esterification
Time (minutes)
Degree of Substitution
(by AGU)
90
120
150
0.2772
1.8412
1.4379
In this study DS results obtained ranged from
0.1669 - 1.8412. Where the highest DS of 1.8412
comes from carboxymethyl starch with 6 grams of
monochloroacetate weight. In the manufacture of
carboxymethyl starch when monochloroacetate is
added too little so that the substituted is too little and
will cause a reaction that occurs less than the
maximum, whereas if the monochloroacetate added
too much will react with NaOH to form Sodium
glycolic acid. This is because in the starch
carboxymethyl synthesis reaction using the
Williamson reaction is carried out in the presence of
a strong base to increase the nucleophilicity of the
hydroxyl group and to help breakdown starch
particles, but side reactions can also occur with
sodium hydroxide, producing glycols (Lawal et al.,
2007).
3.5 SEM Analysis Results
SEM testing was carried out on breadfruit starch and
carboxymethyl starch with the highest degree of
substitution which was in the 6 grams MCA
treatment and 120 minutes reaction time as in Figure
6 and Figure 7.
Figure 6: Surface Morphology of Breadfruit Starch
(Magnification 2500x).
Figure 7: Morphology of Carboxymethyl Starch Surface
(Magnification 2500 x).
SEM analysis is performed to see the
morphology of the modified starch compounds
obtained. In this study, the SEM test was only
performed on carboxymethyl starch with the highest
DS, carboxymethyl starch by adding 6 grams of
MCA weight with a reaction time of 120 minutes,
with an enlarged image of 2500 times. The surface
shape of breadfruit starch at a magnification of 2500
times (figure 6) can be seen clearly that breadfruit
starch consists of finely spaced granules and oval or
egg granules as reported by (Ahmad et al., 1999) and
the carboxymethyl starch surface shape (figure 7)
showing a rougher surface shape because there are
granules around it, with a smaller distance so it
looks like it blends in comparison with the original
breadfruit starch.
4 CONCLUSIONS
From the results of the research that has been done,
the following conclusions can be drawn:
1. The carboxymethyl starch synthesis process uses
2 steps, namely the alkalization process and the
carboxymethylationprocess. The optimum
Carboxymethyl Starch Synthesis from Breadfruit Starch (Artocarpus Communis) through Esterification Reaction with Monochloro Acetate
147

conditions for the 6 grams monocloroacetate
weight gain treatment with an etherification
reaction time of 120 minutes showed a
substituted degree of 1.8412.
2. SEM analysis results show starch surface shape
is smoother, round with a large cavity. While the
carboxymethyl starch surface shape is coarser,
granules have granules around it, have a smaller
distance so it looks like they are fused.
REFERENCES
Ahmad, F. B., Williams, P. A., Doublier, J. L., Durand, S.,
& Buleon, A. (1999). Physico-chemical
characterisation of sago starch. Carbohydrate
Polymers, 38(4), 361–370.
Chen, J., & Jane, J. (1994). Preparation of granular cold-
water-soluble starches by alcoholic-alkaline treatment.
Cereal Chem, 71, 71: 618 – 622.
El-Hamshary, M.G. Fouda, M., MeeraMohideen, El-
Newehy, H. M., Al-Deyab, S. S., & Abdel-Megeed, A.
. (2014). Synthesis and Antibacterial of
Carboxymethyl Starch-Grafted Poly(vinyl imidazole)
Against Some Plant Pathogens. Elsevier.
Fachrudin, R. (2013). Produksi Pati Na-Karboksimetil
dengan Perbedaan Tipe Pengadukan padaTahap
Alkalisasi. IPB Pres.
Kooijman, M., Ganzeveld, J., Manurung, R., & Heeres, H.
(2003). Experimental studies on carboxymethylation
of arrowroot starch in isopropanol-water media.
Starch, 55, 495 – 503.
Lawal, O., Lechner, M., & Kulicke, W. (2007). Single and
multistep carboxymethylation of water yam
(Dioscoreaalata) starch: synthesis and characterization.
Int J BiolMacromol, 429 – 435.
Liu, Q. (2005). Understanding starches and their role in
foods.in: Food Carbohydrates: Chemistry, Physical
Properties, and Applications. Taylor & Francis.
Nurafrida. (2011). Pembuatan Carboxymethil Strach Graft
Polyacrylamide dan Karakterisasinya.
Ochoa, N. (2013). Modified Cassava Starches as Potential
Corrosion Inhibitors for Sustainable Development.
Pitojo, S. (1992). Budidaya Sukun. Kanisius.
Ragheb, A., El-Sayiad, H., & Hebeish, A. (n.d.).
Preparation and characterization of carboxymethyl
starch (CMS) products and their utilization in textile
printing. Starch, 49(Starch), 238 – 245.
Rincom, A.M., & Fanny, C. P. (2004). Physicochemical
properties of Venezuelan breadfruit (Artocarpus
altilis) starch. Arch Latinoam Nutr, 54(4), 449–456.
Sangseethong, K., Ketship, S., & Sriroth, K. (2005). .
2005. The role of reaction parameter on the
preparation and properties of carboxymethyl cassava
starch. Starch., 57, 345 – 359.
Shah, U., & Augsburger, L. (2002). Multiple sources of
sodium starch glycolate, NF: evaluation of functional
equivalence and development of standard performance
tests. Pharm Develop Technol, 7, 345 – 359.
Widowati, S. (2003). Prospek Tepung Sukun Untuk
Berbagai Produk Makanan Olahan Dalam Upaya
Menunjang Diversifikasi Pangan
.
Wurzburg, O. (1989). Modified Starches: Properties and
Uses. CRC Press.
Zhang, B. (2012). Synthesis and characterization of
carboxymethyl potato starch and its application in
reactive dye printing. Elsevier Inc.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
148
