
The Effect of Methyl Oleate Variation as a Template in Synthesis of
Silica Mesoporous using Tetraethylorthosolocate (TEOS)
Silvia Afriani Sitinjak and Andriayani
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan, Indonesia
Keywords: Mesoporous Silica, Methyl Oleate, Template, BET.
Abstract: Silica material synthesis has been carried out using tetraethylortosilicate (TEOS) as a source of silica,
methyl ester oleate as a template, 3-aminopropyltrimethoxysilane as a co-structure directing agent (CSDA),
and deionized water as the solvent. The synthesis of silica material was made by varying the mass of methyl
ester oleate, namely 3.7358 g, 4.2695 g, 4.8032 g, 5.3369 g, and 5.8706 g. The TEOS and APMS mixture
was added to a mixture of methyl ester oleate, 0.1 M HCl, and deionized water and then stirred at room
temperature for 2 hours. The mixture was then cooked in an oven at 80
ο
C for 72 hours. Separation of the
product is done by centrifugation, The resulting solid is dried and then calcined at 550
ο
C for 6 hours. The
XRD analysis results of the product showed diffraction peaks which widened at 12
ο
to 30
ο
which indicated
that the resulting material was amorphous. The FT-IR spectrum shows the presence of Si-OH and Si-O-Si
groups which are characteristic of silica material. SEM analysis shows the existence of hollow particles with
non-uniform size. Nitrogen isotherm (BET) adsorption shows type IV isotherm curves and H1 type
hysteresis loops with uniform pore sizes on the variation of methyl oleate 4.8032 g, which is 2.28 nm. This
identified that the optimum conditions for the synthesis of silica mesoporous material with the methyl ester
oleate template had been achieved.
1 INTRODUCTION
Porous material according to IUPAC can be
classified into three categories based on pore
diameter, namely: (i) micropore (d <2 nm), (ii)
mesoporous (2 nm <d <50 nm), (iii) macropore (d>
50 nm) , and (iv) megapories (> 7500 nm). The
development of the synthesis, characterization, and
application of porous materials has long been carried
out due to its wide use in adsorption, drug delivery,
separation, catalysts, and sensors. Modifications to
the synthesis of porous materials are still being
carried out for the development of material
structures such as porosity and pore diameter.
Silica mesoporous material can be synthesized
by adding templates in the form of anionic, cationic,
or non-ionic surfactants, or non-surfactant
tempalates, where the surfactant charge is based on
the head group load. Pore diameter can be controlled
by changing the molecular carbon chain length of
the template. Templates are used as molds
(auxiliaries and guides) in the formation of pores,
where primary colloidal particles will fill the gaps
between the template arrangements, so that when the
template is removed from silica particles a hollow
particle is formed. Templates can form pores due to
the presence of micelles from the surfactant. Silicate
precursors are formed around the surfactant micelles
by the crystallization process and then form
amorphous silica polymers that surround the
micelles. The micelles are then removed by
calcination, thus leaving a cavity which is then
referred to as a pore.
Research on synthetic silica material using
anionic surfactant templates is undergoing
development. (Yokoi et al., 2006) Synthesized silica
material using sodium laurate and 3-
aminopropyltriethoxysilane (APS) co-structure
directing agent (CSDA). The results show that with
the increase in APS mass used, the pore diameter
decreases from 4.0 nm to 3.3 nm. (Han et al., 2011)
Synthesize mesoporous silica with oleic acid
tempalte with the addition of ethanol as a solvent.
The results show that the more ethanol used, the
pore diameter decreases regularly. (Wan & Zhao,
2007) Synthesized silica mesoporous material using
sodium n-lauroylsarcosin (Sar-Na) with variations in
HCl mass. The results show that the decrease in pH
102
Afriani Sitinjak, S. and Andriayani, .
The Effect of Methyl Oleate Variation as a Template in Synthesis of Silica Mesoporous using Tetraethylorthosolocate (TEOS).
DOI: 10.5220/0010137000002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 102-106
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

causes an increase in pore size from 3.1 nm to 3.3
nm. (Tsai et al., 2011) Synthesized mesoporous
silica nanoparticles (MSNs) with ethylene glycol
phosphate monoester surfactants (PMES) with
variations of 3-aminopropyltrimethoxysilane
(APTMS). The results show that with increasing
APTMS ratio, pore diameter will increase and
decrease regularly. (Andriayani et al., 2018)
synthesized silica material with sodium ricinoleate
with 0.1M HCl variation producing a pore size of
2.2-3.8 nm, and examined the effect of variations in
HCl concentrations in the synthesis of mesoporous
silica materials using methyl ester ricinoleate as a
template (Andriayani et al., 2018). Although
research on the synthesis of mesoporous materials
with anionic surfactant templates continues to
develop, reports of the use of methyl oleate as
templates have not been conducted so far, so that
optimum conditions for obtaining silica mesoporous
materials with good porosity and have a uniform
pore distribution.
Methyl oleate is clear yellow and has nineteen
carbon chains, an ester of oleic acid. Synthesized
through the reaction of esterification with methanol
and catalyst, both acid catalyst or base catalyst. (Ahn
et al., 2012) Synthesized graphene in which methyl
oleate epoxy from methyl oleate to form oleo-
graphene oxide (oleo-GO) which can dissolve in
water, thus forming nanocomposite graphene. (Bao
et al., 2017) esterified oleic acid to methyl oleate
using Zr-SO
3
H @ CMC catalyst which was used as
biofuel.
Based on the above explanation, researchers are
interested in developing research on synthetic silica
mesoporous materials with variations in the number
of methyl oleate templates. The number of templates
plays a role in determining the characteristics of
silica mesoporous material. A high amount of
surfactant will increase the formation of micelles, so
that it will produce more pores. However, if the
amount of surfactant is excessive, it will produce
more micelles and large pores and cause the formed
silica matrix to become brittle (easily broken), so
that optimum conditions will be sought.
2 MATERIALS AND METHODS
2.1 Materials
The materials used in this study include : Oleic
Acid, tetraetilorthosilicat (TEOS), 3-
aminopropiltrimetoksisilan (APMS), HCl 0.1 M,
Deionized Water,n-hexane, Calcium
Chloride(CaCl
2
), methanol, Methyl Oleate ,sulfuric
acid (H
2
SO
4
).
2.2 Methods
Esterification of Oleic Acid
In a three neck flask (250 ml), oleic acid (40 g ;
0.134 mol), dried methanol (85.76 g; 2.68 mol), and
H
2
SO
4(p)
(0.8 g) catalyst, then heated in reflux circuit
at a temperature of 60-70
ο
C while stirring for 3-4
hours. The reaction results obtained added aquadest.
After that the methyl ester formed is extracted with
n-hexane. Oleic oil methyl esters obtained were
analyzed using GC-MS.
Synthesis of Mesoporous Silica
Into the beaker glass, 3.7358 g of methyl ester oleate
(0.01126 mol) are added, then add 100 ml of
demineralized water and stir for 20 minutes. Then
add 30 ml of 0.1 M HCl while stirring at room
temperature for 5 minutes. Then a mixture of 3.74 g
TEOS (0.018 mol) and 1 g APMS (0.00559 mol)
was made and stirred for 5 minutes in a closed
condition. The TEOS and APMS mixture was added
to the mixture of methyl ester oleate, demineralized
water, and HCl and then stirred for 2 hours. Then
aging in an oven at 80
ο
C for 72 hours. The product
is separated using a centrifugator and washed with
demineralized water and then dried. The resulting
solid was calcined at 550
ο
C for 6 hours. The results
obtained were characterized by FT-IR, XRD, SEM
and BET analysis.
Table 1: Condition reaction of synthesis mesoporous silica.
Treatment TEOS
(mol)
Methyl Oleate
(mol)
Methyl Oleate
(gram)
APMS
(mol)
HCl
(ml)
Stirrer Time
(hours)
Run-1 0.018 0.00126 3.7358 0.00559 30 2
Run-2 0.018 0.0144 4.2695 0.00559 30 2
Run-3 0.018 0.0164 4.8032 0.00559 30 2
Run-4 0.018 0.0180 5.3369 0.00559 30 2
Run-5 0.018 0.0198 5.8706 0.00559 30 2
The Effect of Methyl Oleate Variation as a Template in Synthesis of Silica Mesoporous using Tetraethylorthosolocate (TEOS)
103
3 RESULTS AND DISCUSSION
3.1 GC-MS Analysis of Methyl Oleate
The esterification results of oleic acid were analyzed
using GC-MS to see the purity of the methyl oleate
produced. The results of the GCMS analysis showed
that the percentage of methyl oleate was the largest
at 86.77%.
3.2 Synthesis of Mesoporous Silica
Into the beaker glass, methyl ester oleate is added
with demineralized water. Then added 0.1 M HCl
then stirred at room temperature for 1 hour. The
mixture of terraethylortosilicate (TEOS) and 3-
aminopropyltrimethoxysilane (APMS) was stirred
for 5 minutes, then the mixture of
terraethylortosilicate (TEOS) and 3-
aminopropyltrimethoxysilene (APMS) was added to
the beaker glass containing a mixture of methyl ester
oleate, HCl, and water in the demineralized water
room temperature for 2 hours. Aging was carried out
in the oven at 80
ο
C for 72 hours. The product is
separated by centrifugation and then dried and then
calcined at 550
ο
C for 6 hours. The result was 0.7873
g, 0.7932 g, 1.0904 g, 0.8576 g, and 0.8963 g.
3.3 FT-IR Analysis of Material
Mesoporous Silica
Figure 1: FT-IR analysis of mesoporous silica.
The picture above shows that the methyl ester oleate
used as a template was lost during calcination. This
can be seen from the absence of C = O uptake in the
range 1725 cm
-1
and C = C uptake in the range
1680-1640 cm
-1
. It can also be seen from the figure
above that all mesoporous materials with the
addition of various oleic methyl esters show the
absorption peak between 3448.72 cm
-1
(broad) given
by the OH group strain (ʋ
as
Si-OH), whereas at
802.39 and 794.67 cm
-1
is caused by the presence of
Si-OH symmetrical groups (ʋ
s
Si-OH). Other
absorption peaks were seen at 1095.57 and 1087.85
cm
-1
(strong) given by the strain Si-O-Si (ʋ
as
Si-O-
Si), while at 462.92-455.20 cm
-1
caused by the
symmetric group Si-O-Si (ʋ
s
Si-O-Si).
3.4 Difraction of XRD Mesoporous
Silica
Figure 2: Difractogram for mesoporous silica.
Table 2: Functional group of mesoporous silica.
Silica Material Functional Group Wavelength
Si-OH
(as)
Si-O-Si
(as)
Si-OH
(s)
Si-O-Si
(s)
Run-1(MO 3.7358 g) 3448.72 794.67 1087.85 462.92
Run-2(MO 4.2695 g) 3448.72 802.39 1085.85 462.92
Run-3(MO 4.8032 g) 2448.72 802.39 1095.57 462.92
Run-4(MO 5.3369 g) 3448.72 794.67 1095.57 470.63
Run-5(MO 5.8706 g) 3441.01 802.39 1087.85 462.92
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
104
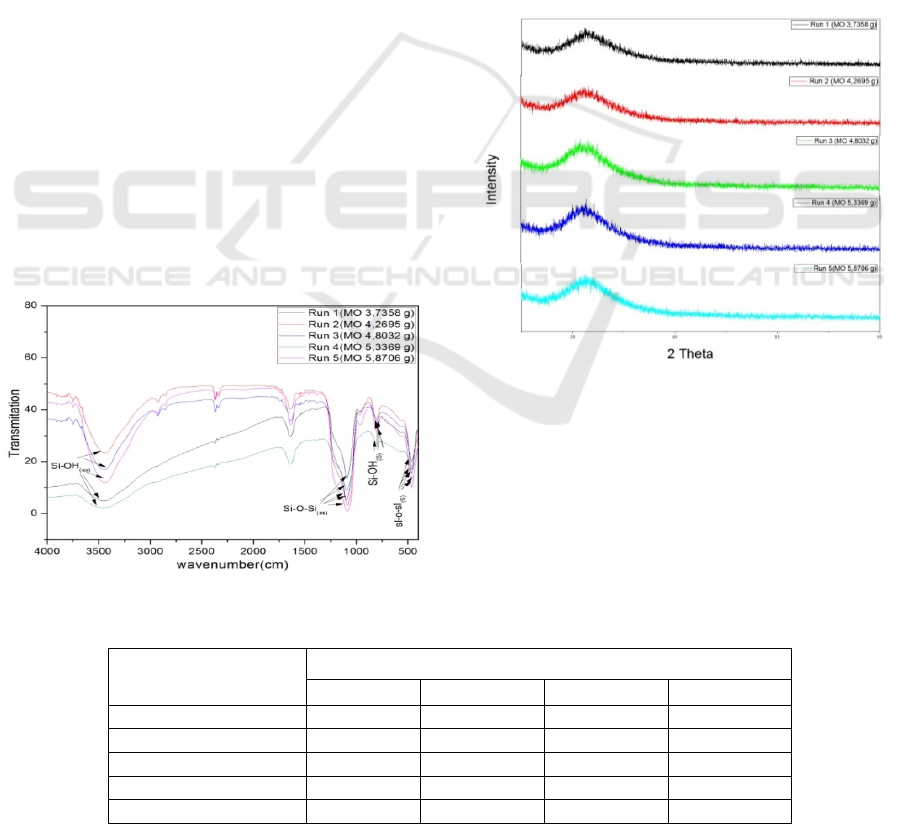
From the picture above can be seen XRD
diffractogram at an angle of 2θ which widens
between 120
ο
to 38
ο
. Diffraction peaks that widen
from silica material in run-1, run-2, run-3, run-4, and
run-5 are 22.32, 23.07, 21.66, 22.61, and 22.28
which shows that the material produced is silica and
has an amorphous structure. This is consistent with
the data found in the literature (Andriayani et al.,
2013; Zhao et al., 2014).
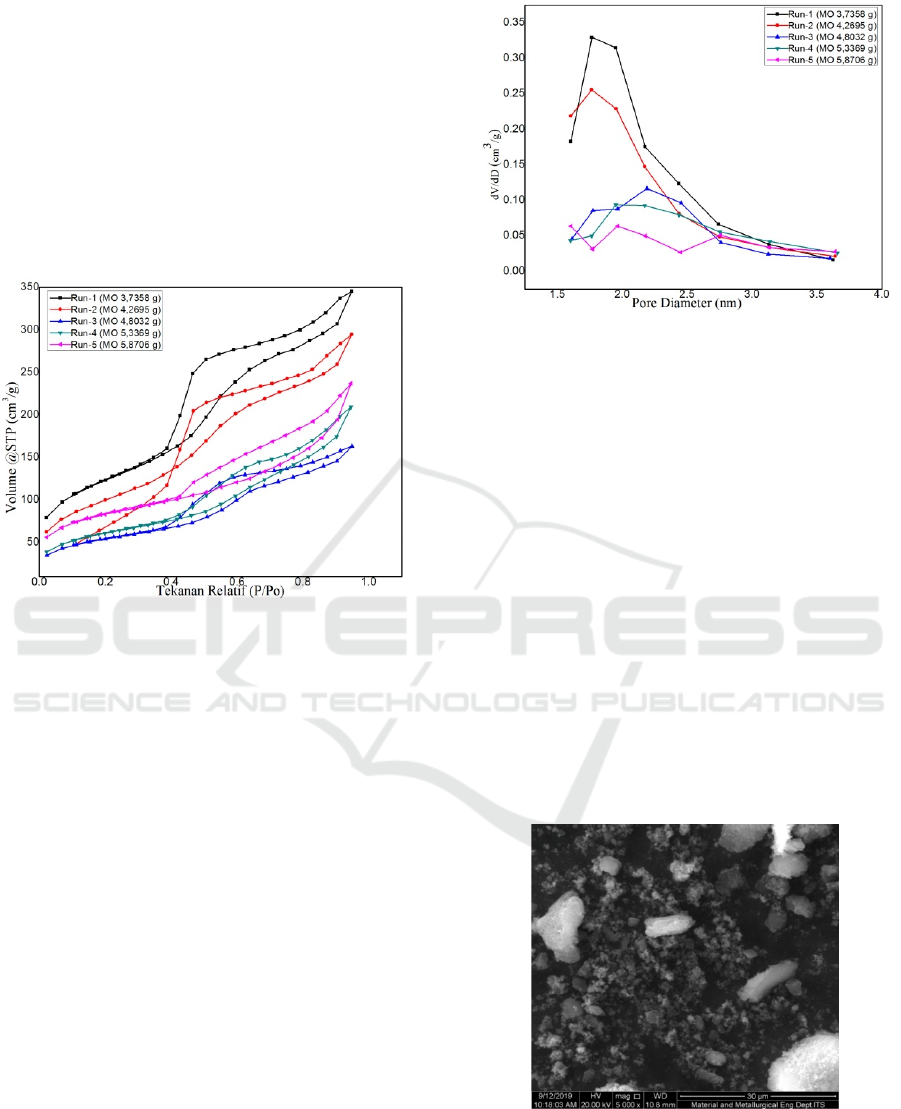
3.5 Isoterm Adsorbsi-desorbtion
Figure 3: Isoterm adsrobtion-desorbtion of mesoporous
silica.
Graphs of run-1, run-2, run-3, run-4, and run-5 silica
material isotherm silica material show loop
hysteresis at relative pressure (P/P
o
) between 0.45-1
and physisorption isotherm type IV according to the
IUPAC classification (Gregg & Sing, 1982). The
hysteresis loop shape in run-1, run-2, and run-3
silica material is H1 type which shows that porous
material is cylindrical, such as pore ducts or
agglomerates of solids with coarse homogeneous
fields (Roque-Malherbe, 2007). Whereas run-4 and
run-5 has hysteresis loops of type H3, namely the
existence of nonrigid aggregates of particles such as
plates which have pore-shaped gaps.
Pore size distribution of the resulting silica
material can be seen in the following figure:
Figure 4: Pore size distribution for mesopoorous silica.
In Figure 3.4, several different peaks of each of the
silica material produced indicate that the pores
formed are not uniform. The resulting pore diameter
starts from 1.59 nm - 3.65 nm, so it can be classified
into micropore and mesoporous sizes. The dominant
pore size for variations in the addition of methyl
ester oleate to run-1, run-2, run-3, run-4, and run-5
are 1.77 nm, 1.77 nm, 2.18 nm and 2.45 nm, 1.96
nm, and 2.00 nm and 2.769 nm.
3.6 Microskop Electron Scanning
(SEM)
Based on the BET test, it was found that run-3 silica
material has an even distribution of pore size, so to
find out the morphology of run-3 silica material is
done with SEM photographs with various
magnifications of 5,000 and 20,000 times. The SEM
photo is shown in the image below:
Figure 5(A): Magnification 5,000 times.
The Effect of Methyl Oleate Variation as a Template in Synthesis of Silica Mesoporous using Tetraethylorthosolocate (TEOS)
105
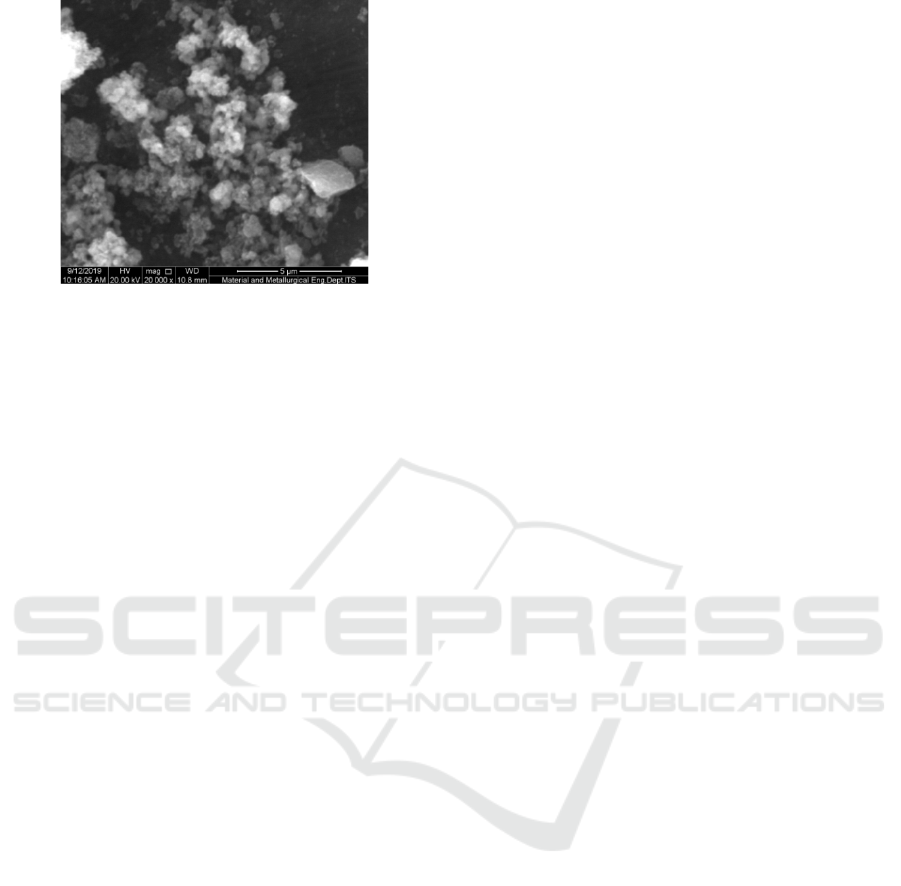
Figure 5(B): Magnification 20,000 times.
SEM photos in Figure (A) with a magnification of
5,000 times, it appears that the shape of the particle
is not clear and only looks uneven chunks and there
are small aggregates that are dispersed on the
surface of the material, while at magnification
20,000 times (figure B ) have started to clearly see
diverse particle shapes, such as large lumps and
spherical shapes. And inside the particle there is a
gap.
4 CONCLUSIONS
The characteristics of the mesoporous silica material
produced, namely the FT-IR spectrum shows the
presence of silanol groups (Si-OH) and siloxane
groups (Si-O-Si) which are characteristic of silica
material. XRD diffractogram shows the diffraction
peaks widened at angle of 2θ between 20-40
ο
, so that
the silica material formed is silica mesoporous. SEM
photos show the dispersed aggregates on the surface
of the material in a non-uniform pore size condition.
The isotherm nitrogen adsorption descent with the
BET method shows a type IV isotherm curve that is
an isotherm type for mesoporous material and has
H1 hysteresis loop type in run-1, run-2, and run 3
silica material. While run-4 and run- silica material 5
has hysteresis loop type H3. The dominant pore
diameter sizes are 1.77 nm, 1.77 nm, 2.18 nm and
2.45 nm, 1.96 nm, and 2.00 nm and 2.769 nm,
respectively.
REFERENCES
Ahn, B. K., Sung, J., Li, Y., Kim, N., Ikenberry, M.,
Hohn, K., Mohanty, N., Nguyen, P., Sreeprasad, T. S.,
Kraft, S., Berry, V., & Sun, X. S. (2012). Synthesis
and characterization of amphiphilic reduced graphene
oxide with epoxidized methyl oleate. Advanced
Materials. https://doi.org/10.1002/adma.201104080
Andriayani, A., Sembiring, S. B., Aksara, N., & Sofyan,
N. (2013). Synthesis of Mesoporous Silica from
Tetraethylorthosilicate by Using Sodium Ricinoleic as
a Template and 3-Aminopropyltrimethoxysilane as
Co-Structure Directing Agent with Volume Variation
of Hydrochloric Acid 0.1 M. Advanced Materials
Research, 789, 124–131.
https://doi.org/10.4028/www.scientific.net/AMR.789.
124
Andriayani, Nainggolan, H., Taufik, M., Simamora, S., &
Sofyan, N. (2018). The effect concentration of
tetraethylorthosilicate and variation HCl 0.1M for
synthesis mesoporous silica using oleic acid as
template and 3-aminopropyltrimethoxysilane as co-
structure directing Agent. Journal of Physics:
Conference Series, 1116(4), 0–8.
https://doi.org/10.1088/1742-6596/1116/4/042006
Bao, Y., Wang, T., Kang, Q., Shi, C., & Ma, J. (2017).
Micelle-template synthesis of hollow silica spheres for
improving water vapor permeability of waterborne
polyurethane membrane. Scientific Reports,
7(December 2016), 1–14.
https://doi.org/10.1038/srep46638
Gregg, S. J., & Sing, K. S. (1982). Adsorpsi, Surface and
Porosity (2nd ed.). Academic Press.
Han, L., Gao, C., Wu, X., Chen, Q., Shu, P., Ding, Z., &
Che, S. (2011). Anionic surfactants templating route
for synthesizing silica hollow spheres with different
shell porosity. Solid State Sciences.
https://doi.org/10.1016/j.solidstatesciences.2010.05.00
9
Roque-Malherbe, R. M. A. (2007). Adsorption and
Difussion in Nanoporous Materials. CRC Press Taylor
& Francis Group.
Tsai, C. H., Vivero-Escoto, J. L., Slowing, I. I., Fang, I. J.,
Trewyn, B. G., & Lin, V. S. Y. (2011). Surfactant-
assisted controlled release of hydrophobic drugs using
anionic surfactant templated mesoporous silica
nanoparticles. Biomaterials.
https://doi.org/10.1016/j.biomaterials.2011.04.077
Wan, Y., & Zhao, D. (2007). On the controllable soft-
templating approach to mesoporous silicates. In
Chemical Reviews. https://doi.org/10.1021/cr068020s
Yokoi, T., Yoshitake, H., Yamada, T., Kubota, Y., &
Tatsumi, T. (2006). Amino-functionalized mesoporous
silica synthesized by an anionic surfactant templating
route. Journal of Materials Chemistry.
https://doi.org/10.1039/b516863e
Zhao, Q., Mao, Y., Yan, L., Lu, L., Jiang, T., & Yin, H.
(2014). Stability of Y/MCM-48 composite molecular
sieve with mesoporous and microporous structures.
Journal of Asian Ceramic Societies, 2(4), 347–356.
https://doi.org/10.1016/j.jascer.2014.07.003
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
106
