
First Record of Two Species of Cobbonchus Andrassy, 1958
(Nematoda: Cobbonchidae) from South Kalimantan
Abdul Gafur
Department of Biology, Lambung Mangkurat University, Jalan Ahmad Yani Km. 36, Banjarbaru, Indonesia
Keywords: Biodiversity, Description, Identification, Mononchida, Predatory nematodes.
Abstract: For several decades soil nematode community structure has been used as bioindicator of soil conditions.
Because the accuracy of the assessment necessitates proper identification of existing taxa, the potential use
of nematode as bioindicator can only be realized in places where most soil nematode species have been well
identified. Previous surveys of soil nematodes in South Kalimantan (Borneo) left some specimens that were
identified as belonging to the genus Cobbonchus Andrassy, 1958, but the species was unknown. In the
present study, as a part of the South Kalimantan Nematode Biodiversity Project, the specimens were further
examined and identified as Cobbonchus collaris Andrassy, 1985 and Cobbonchus indicus Baqri, Baqri &
Jairajpuri, 1978. Despite some differences, morphological characters of the South Kalimantan specimens
generally conformed the previously published descriptions of the species. The deviations were considered
intraspecific variations that may lead to redescriptions of the corresponding species. This is the first record
of occurrence of the two species in South Kalimantan that provides new data on the geographic distribution
of the genus and species.
1 INTRODUCTION
Nematodes, in addition to prokaryotes, are the major
components of biodiversity in soil ecosystems. Not
only nematodes are the most abundant metazoans in
soils (Sohlenius, 1980; Bongers and Bongers, 1998),
nematode community is also characterized by a high
species diversity (Yeates, 1979) that most trophic
levels of the soil food web are occupied by
nematodes (Yeates et al., 1993). Therefore,
nematode diversity is indicative of general
biodiversity in the soil (Yeates and Bongers, 1999).
It has been shown that nematodes play crucial
roles in fundamental ecological processes in soils
and therefore they are correlated with soil functional
parameters and reflects soil functioning (Bongers
and Bongers, 1998; Ekschmitt et al., 2001; Hodda,
Peters and Traunspurger, 2009). This is the basis for
the using of nematode community structure as
bioindicator of soil ecological condition (Bongers,
1990).
Although in general the using of nematodes as
bioindicator of soil condition does not require
identification to species level (Bhusal et al., 2014),
more information would be obtained with species-
level discrimination (Ferris and Bongers, 2009).
Species-level identification is also necessary to
further understand the role of nematodes in soil
processes and thus in ecosystem resilience (Yeates,
2003). In regions where the nematofauna has not
been explored, such as South Kalimantan (Borneo),
inventory of soil nematodes is a prerequisite for the
using of nematodes as bioindicators. Therefore,
attempts should be made to reach species-level
identification.
In most soils mononchids comprise important
component of the nematode communities. The
predatory nematodes can be found in all kind of
soils, and in much greater number in undisturbed
soils (Ahmad and Jairajpuri, 2010).
Specimens of mononchids were collected during
a nematode ecology survey held in South
Kalimantan in 2005. The specimens were deposited
in the Nematode Collection of the Laboratory of
Biosystematics, Faculty of Mathematics and Natural
Sciences, Lambung Mangkurat University. These
were identified as belong to the genus Cobbonchus
Andrassy, 1958. However, the identification was not
continued to species.
In the present study the mononchid specimens
were further examined and described herein as
Cobbonchus collaris Andrassy, 1985 and
90
Gafur, A.
First Record of Two Species of Cobbonchus Andrassy, 1958 (Nematoda: Cobbonchidae) from South Kalimantan.
DOI: 10.5220/0010136800002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 90-95
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
Cobbonchus indicus Baqri, Baqri & Jairajpuri, 1978.
These are the first record of existence of the two
species in South Kalimantan, and presumably in the
Island of Kalimantan (Borneo) or even in Indonesia.
2 MATERIALS AND METHODS
In the 2005 survey specimens of mononchid
nematodes were collected by the author from
Gambut Subdistrict, Banjar District, South
Kalimantan.
The nematodes were killed and fixed in hot 4%
formalin and were transferred to pure glycerine
using rapid method of Seinhorst (1959). The
specimens were then mounted in Cobb double
coverslip slides.
Morphology of the specimens were examined
under a compound microscope (Nikon E100). A
mirrorless camera was attached to the eyepiece and
connected to a laptop. Measurements were taken
using Camera Measure Version 2.1.3.250 software
(© e2eSoft) that has been calibrated by an ocular
micrometer.
3 RESULTS AND DISCUSSION
3.1 Cobbonchus collaris Andrassy, 1985
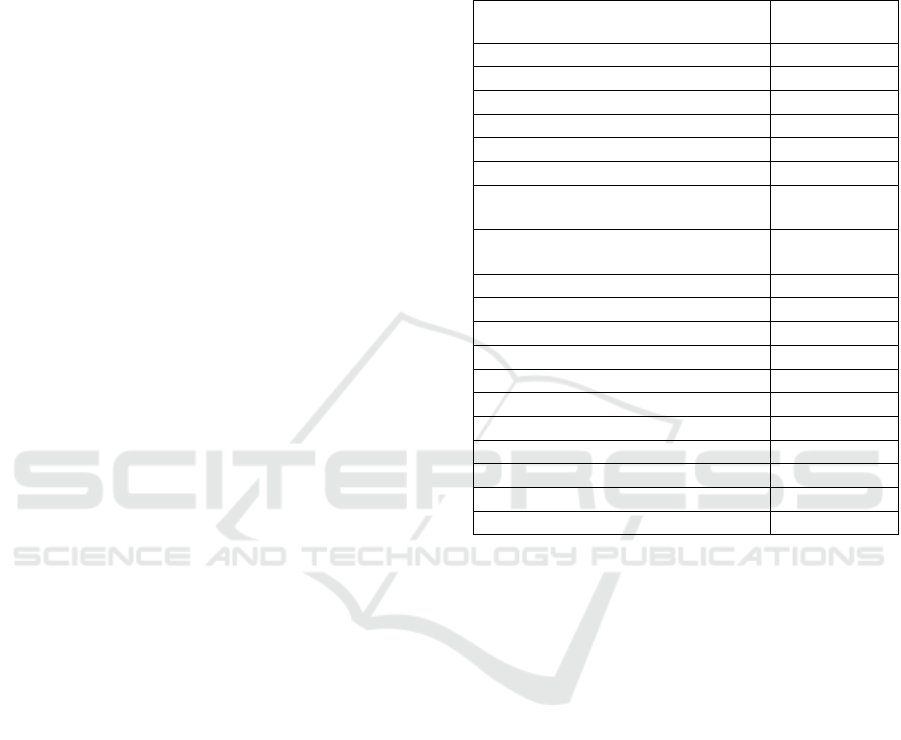
3.1.1 Female (Fig. 1)
Measurements: see table 1.
Body slender, ventrally arcuate after fixation,
posterior part more curved than anterior. Cuticle
smooth. Lip region not offset from the rest of the
body, 24 μm wide. Buccal cavity thick-walled with a
narrow base, 36x15 μm, 1.5 times longer than
cephalic diameter. Stoma formed with three teeth of
nearly equal size. Dorsal tooth medium, located in
anterior half of buccal cavity; its pointed apex at
71% of buccal cavity length from base. Two
subventral teeth in posterior half of buccal cavity,
anteriorly directed apexes at 45% of buccal cavity
length from base. Pharynx cylindroid, muscular,
26% of body length. Excretory pore indistinct.
Pharyngointestinal junction non-tuberculate.
Genital system amphidelphic, anterior and
posterior branch equal in length. Vulva slightly
posterior to mid body, lips not protruding. Vagina 13
µm, about 1/3 of corresponding body width.
Distance vulva-anus 8.5 times longer than tail.
Rectum 19 µm, shorter than anal body width. Tail
short 23 µm, about 2.2 anal body width long,
uniformly tapering, with well developed caudal
glands arranged in tandem and terminal opening.
Table 1: Morphometrics of Cobbonchus collaris from
South Kalimantan.
Characters
C. collaris
(
n=1
)
Bod
y
len
g
th
(
L
)
1305
Max. bod
y
width
(
BW
)
38.2
Li
p
re
g
ion len
g
th 8.0
Li
p
re
g
ion width 23.6
Buccal cavit
y
len
g
th 36.5
Buccal cavit
y
width 15.0
Dorsal tooth apex as % of buccal
cavit
y
len
g
th from
b
ase
71.2
Subventral teeth apices as % of
b
uccal cavit
y
len
g
th from
b
ase
44.9
Phar
y
nx len
g
th 346.9
Tail len
g
th
(
T
)
56.9
Rectum len
g
th 19.5
Anal bod
y
width
(
ABW
)
26.2
a
(
L/BW
)
34.2
b
(
L/distance anterior to 3.4
c
(
L/T
)
22.9
c’
(
T/ABW
)
2.2
V
(
L/distance anterior to vulva in %
)
58.6
%Phar
y
nx/L 26.6
Rectum/ABW 0.7
All measurements are in µm.
3.1.2 Male
Male not found.
3.1.3 Locality and Habitat
Specimens were recovered from a peatland covered
by ferns and Malaleuca leucadendra in Gambut
Subdistrict, Banjar District, the Province of South
Kalimantan, Indonesia.
3.1.4 Remarks
The examined specimen of Cobbonchus from South
Kalimantan population fits well the description of
Cobbonchus collaris by Andrassy (1985), except in
shorter body length (1.3 mm versus 1.6 mm), more
posterior dorsal tooth apex (29% versus 22% of
buccal cavity), shorter oesophagus relative to body
length (27% versus 29-30%), shorter rectum relative
to anal body width ( 70% versus 100%), relative
length of anterior to posterior branch of female
gonads (equal versus posterior shorter).
First Record of Two Species of Cobbonchus Andrassy, 1958 (Nematoda: Cobbonchidae) from South Kalimantan
91
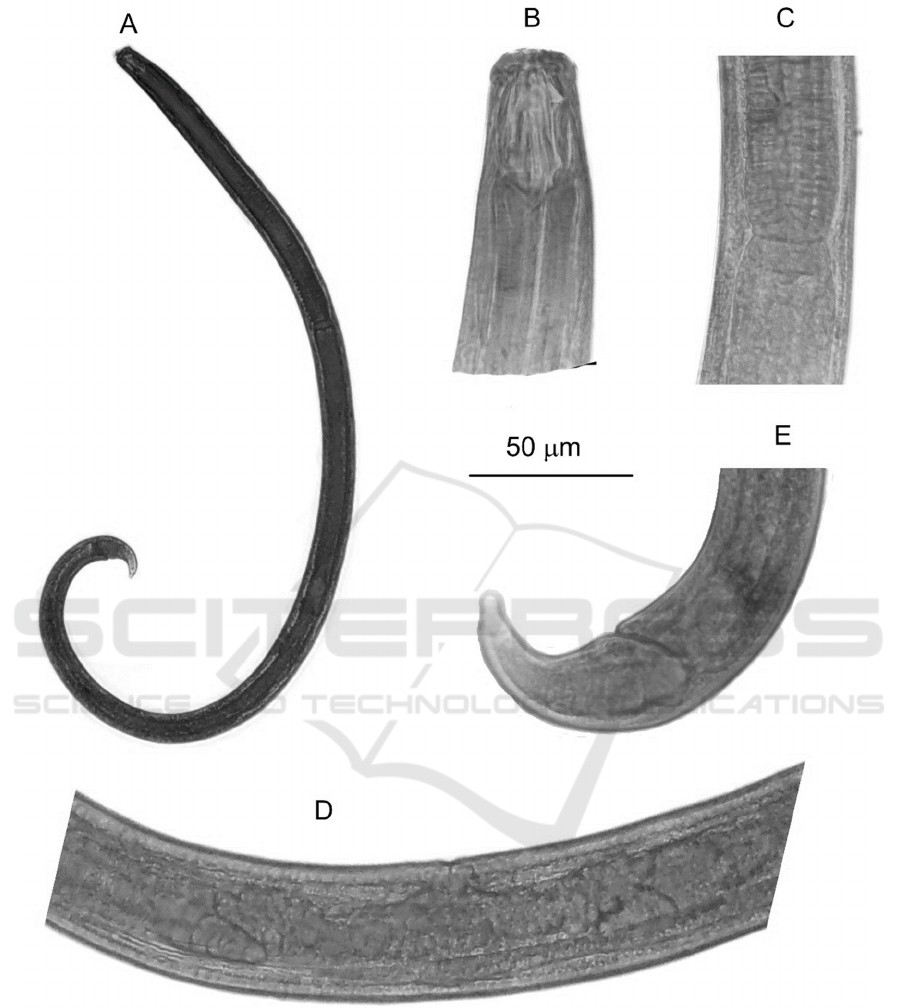
Figure 1: Cobbonchus collaris female from South Kalimantan. A. Whole body; B. Head; C. Pharyngo-intestinal junction;
D. Gonad; E. Tail. Scale bar applies to B-E.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
92
On the other hand, it conforms the description of C.
macrampulla by Orselli and Vinciguerra (2007) in
body length, position of dorsal tooth apex, and
rectum length relative to anal body width.
The uniformly narrowing tail agrees best with
those of C. collaris, C. macrampulla, C. palustris,
and C. radiatus. However, the examined specimen
does not show the large, sclerotized ampulla at the
end of the caudal glands typical of C. macrampulla,
the rounded lip region of C. palustris, and the offset
head of C. radiatus. Furthermore, the caudal glands
of the examined specimen are larger than those of C.
radiatus. However, comparison with C. radiatus can
not be thoroughly made as the description of the
species was based on a single young adult female
(Clark, 1960) and it is considered species inquirenda
(Andrássy, 1985). It is concluded that the examined
specimen belongs to a population of C. collaris and
the differences in some morphometrics are here
considered intraspecific variations.
3.2 Cobbonchus Indicus, Baqri, Baqri and
Jairajpuri, 1978
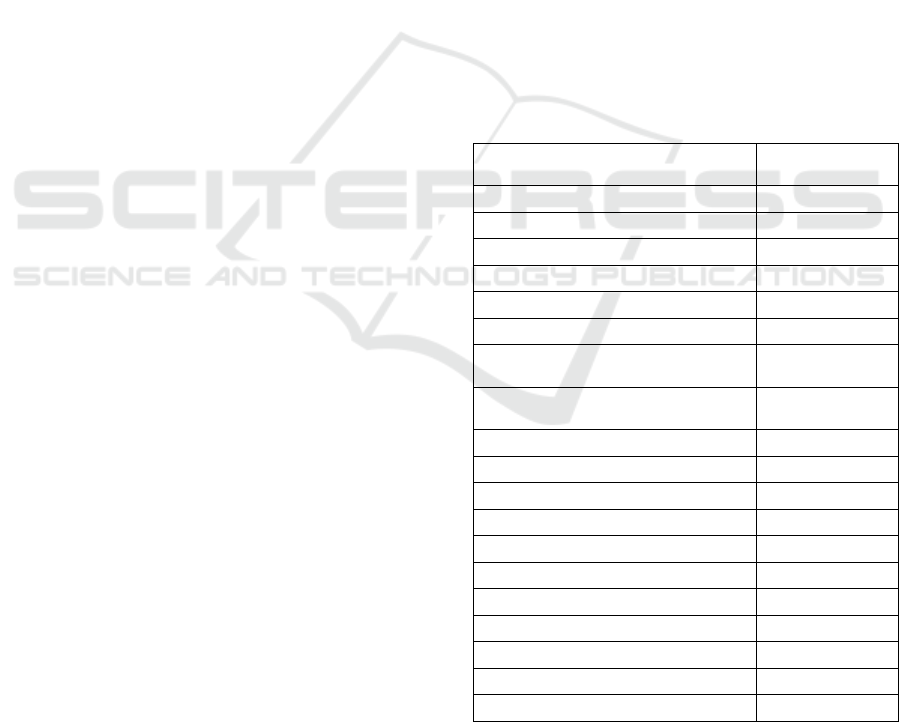
3.2.1 Female (Fig. 2)
Measurements: see table 2.
Body slender, posterior half strongly curved
ventrally after fixation. Head offset. Amphids cup-
shaped, with slit-like apertures close to anterior end
of stoma. Buccal cavity 26x11, anterior end arching
inwards, posterior end somewhat pointed. Dorsal
tooth apex at circa 18 um or 67% from base of
stoma; subventral teeth smaller than dorsal, their
apices at about 9 um or 35% from base of stoma.
Oesophago-intestinal junction non-tuberculate.
Excretory pore indistinct.
Reproductive system amphidelphic. Vulva at
posterior half of body, about two third of body
length. Vagina sclerotized distally, extending
inwards up to half of corresponding body width.
Ovaries reflexed. Tail short conoid, rather bulbous,
slightly ventrally curved, 0.8 anal body-width long.
Rectum equal to or slightly longer than anal body
width. Caudal glands well developed; opening
subdorsal.
3.2.2 Male
Male not found.
3.2.3 Locality and Habitat
Specimens were collected from a peatland covered
by ferns and Malaleuca leucadendra in Gambut
Subdistrict, Banjar District, the Province of South
Kalimantan, Indonesia.
3.2.4 Remarks
The specimens of Cobbonchus examined agree with
general morphology of Cobbonchus indicus as
described by Baqri et al. (1978), except in some
morphometric details. The South Kalimantan
specimens are slightly longer (body length 1.16-1.30
mm versus 1.07 mm). Dorsal tooth apex is more
posterior (65-70% versus 78% from base of stoma).
Tail is shorter (16-24 versus ca 30 µm), mainly
because of the shorter finger-like projection.
Despite the differences, the specimens from South
Kalimantan are considered to belong to a population
of Cobbonchus indicus. This is a new record of
existence of this species in South Kalimantan and
Indonesia, and even outside the type locality in
India.
Table 2: Morphometrics of Cobbonchus indicus from
South Kalimantan.
Characters
C. indicus
(n=3)
Body length (L) 1165 – 1360
Max. body width 30.5 – 41.3
Lip region length 3.7 – 4.7
Lip region width 17.8 – 20.7
Buccal cavity length 25.9 – 27.0
Buccal cavity width 10.5 – 12.6
Dorsal tooth apex as % of buccal
cavit
y
len
g
th from base
64.9 – 70.0
Subventral teeth apices as % of
b
uccal cavity length from base
32.8 – 40.4
Pharynx length 324.9 – 354.4
Tail length (T) 17.5 – 21.9
Rectum length 20.9 – 29.8
Anal body width (ABW) 21.0 – 24.3
a (L/max body width) 32.9 – 38.2
b ( 3.3 – 3.5
c 62.1 – 71.1
c’ 0.8 – 0.9
V 65.4 – 67.4
%Pharynx/L 26.1 – 27.9
Rectum/ABW 1.0 – 1.2
All measurements are in µm.
First Record of Two Species of Cobbonchus Andrassy, 1958 (Nematoda: Cobbonchidae) from South Kalimantan
93
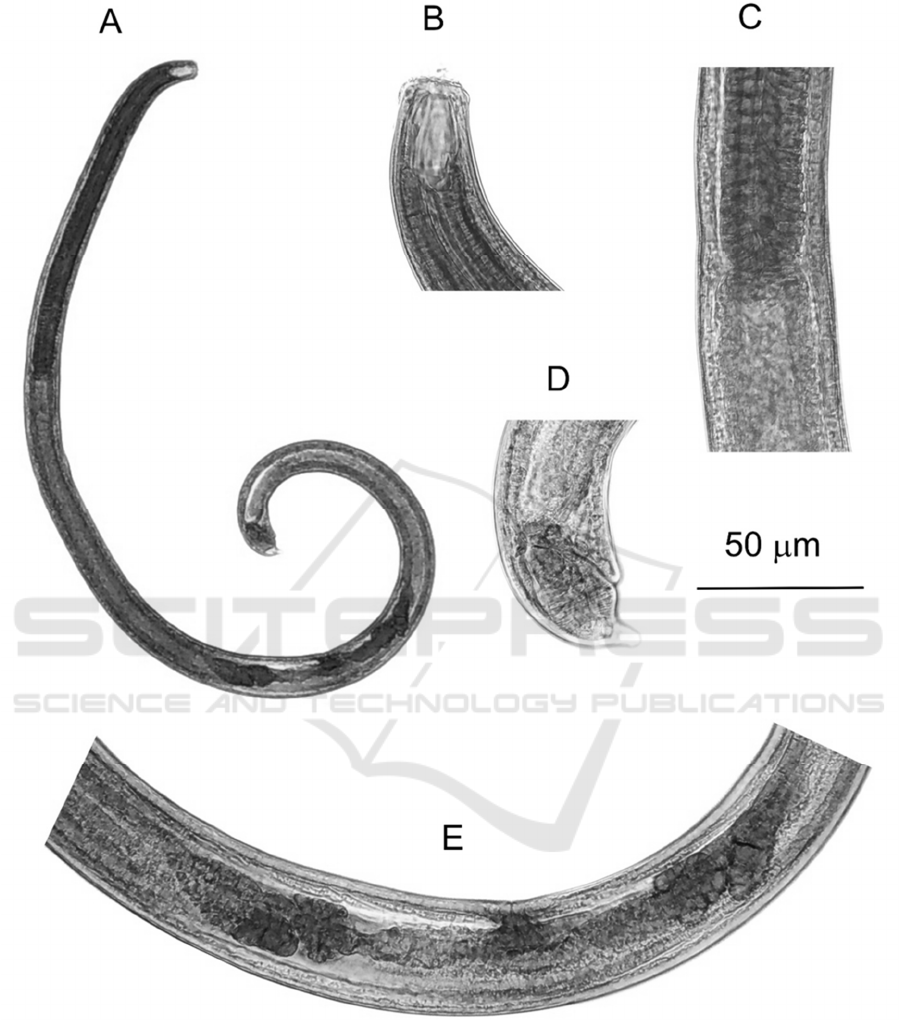
Figure 2: Cobbonchus indicus female from South Kalimantan. A. Whole body; B. Head; C. Pharyngo-intestinal junction; D.
Tail; E. Gonad. Scale bar applies to B-E.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
94

4 CONCLUSIONS
Specimens of Cobbonchus in the nematode
collection of Laboratory of Biosystematics, Faculty
of Mathematics and Natural Sciences, Lambung
Mangkurat University have been identified as
Cobbonchus collaris and Cobbonchus indicus. This
is the first report of existence of the two species in
South Kalimantan and in Indonesia.
ACKNOWLEDGEMENTS
The present study was financially supported by The
Faculty of Mathematics and Natural Sciences
Lambung Mangkurat University.
REFERENCES
Ahmad, W. and Jairajpuri, M. S. 2010. Mononchida,
Mononchida. Leiden: Brill.
Andrássy, I. 1985. ‘On the Genera Mononchus Bastían,
1865 and Prionchuhis (Cobb, 1916) Wu & Hoeppli,
1929 (Nematode: Mononchidae)’, Opuscula Zoologica
Budapest, 21, pp. 9–22.
Baqri, Q. H., Baqri, S. Z. and Jairajpuri, M. S. 1978.
‘Studies On Mononchida XI. Two New Species of
Iotonchus, Cobbonchus indicus n. sp. and Anatonchus
ginglymodontus Mulvey, 1961’, Nematologica, 24,
pp. 436–444.
Bhusal, D. R. et al. 2014. ‘Higher taxa vs. functional
guilds vs. trophic groups as indicators of soil
nematode diversity and community structure’,
Ecological Indicators.
Bongers, T. 1990. ‘The maturity index: an ecological
measure of environmental disturbance based on
nematode species composition’, Oecologia, 83(1), pp.
14–19.
Bongers, T. and Bongers, M. 1998. ‘Functional diversity
of nematodes’, Applied Soil Ecology, 10(3), pp. 239–
251.
Clark, W. C. 1960. ‘The Mononchidae (Enoplida,
Nematoda) of New Zealand III. A review of the genus
Cobbonchus Andrassy, 1958 with descriptions of new
species’, Nematologica, 5(4), pp. 275–284.
Ekschmitt, K. et al. 2001. ‘Nematode community structure
as indicator of soil functioning in European grassland
soils’, European Journal of Soil Biology.
Ferris, H. and Bongers, T. 2009. ‘Indices developed
specifically for analysis of nematode assemblages’, in
Wilson, M. J. et al. (eds) Nematodes as Environmental
Indicators. Wallingford UK: CABI Publishing, pp.
124–145.
Hodda, M., Peters, L. and Traunspurger, W. 2009.
‘Nematode diversity in terrestrial, freshwater aquatic
and marine systems’, Nematodes as environmental
indicators. CAB International, pp. 45–93.
Orselli, L. and Vinciguerra, M. T. 2007. ‘Three new and a
rare species of Mononchida (Nematoda) from
Ecuador’, Journal of Nematode Morphology and
Systematics, 9, pp. 137–146.
Seinhorst, J. W. 1959. ‘A rapid method for the transfer of
nematodes from fixative to anhydrous glycerin’,
Nematologica. Brill Academic Publishers, 4(1), pp.
67–69.
Sohlenius, B. 1980. ‘Abundance, Biomass and
Contribution to Energy Flow by Soil Nematodes in
Terrestrial Ecosystems’, Oikos. WileyNordic Society
Oikos, 34(2), pp. 186–194.
Yeates, G. W. 1979. ‘Soil nematodes in terrestrial
ecosystems.’, Journal of Nematology, 11(3), pp. 213–
229.
Yeates, G. W. et al. 1993. ‘Feeding habits in soil
nematode families and genera-an outline for soil
ecologists.’, Journal of nematology. Society of
Nematologists, 25(3), pp. 315–31.
Yeates, G. W. and Bongers, T. 1999. ‘Nematode diversity
in agroecosystems’, Agriculture, Ecosystems and
Environment.
Yeates, G. W. 2003. ‘Nematodes as soil indicators:
functional and biodiversity aspects’, Biology and
Fertility of Soils, 37(4), pp. 199–210.
First Record of Two Species of Cobbonchus Andrassy, 1958 (Nematoda: Cobbonchidae) from South Kalimantan
95
