
Corrosion Inhibitors Activity of Schiff Base from Condensation of
Ethylenediamine with Furfural from Sugarcane Bagasse
Mimpin Ginting, Dasron Bulolo, Herlince Sihotang
and Indra Masmur
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan, Indonesia
Keywords: Schiff Base, Ethylenediamine, Furfural, Corrosion Inhibitor, Zinc.
Abstract: Schiff Base from condensation of ethylenediamine as the source of primary amine with furfural from
sugarcane bagasse as the source of carbonyl has been synthesized and tested for its corrosion inhibitors
activity. The yield of furfural from 100 g of sugarcane bagasse is 12.6 g. Qualitative analysis shows the
existence of furfural in brick red colour by using aniline : acetic acid (1:1 v/v) as solvent. FT-IR
Spectroscopy shows vibration in wavenumber 1677 cm
-1
as C=O and 1573 cm
-1
as cyclic C=C.
Condensation 9.6 g of furfural with 2.4 g of ethylenediamine in reflux condition for 5 hours using ethanol as
solvent yields 8.64 g (70.55%) of Schiff Base. FT-IR Spectroscopy for Schiff Base analysis shows vibration
of –C=N- in 1647 cm
-1
. The test of corrosion inhibitor activity is by weighting the decrease mass of zinc in
HCl 0.1 N with concentration 7000 ppm for each compound. The efficiency value of corrosion inhibitor are
69.06% for furfural, 53.67% for ethylenediamine, and 82.20% for synthesized Schiff Base.
1 INTRODUCTION
Sugarcane bagasse is a solid part of cane from
extracted cane stem. Sugarcane bagasse consists of
C (carbon) 47%, H (hydrogen) 6.5%, O (oxygen)
44%, and ash 2.5%. Based on Pritzelitz formula
(Hugot, 1986) every kilogram of sugarcane bagasse
contains 2.5% sugar which produce 1825 kcal/kg.
Composition of sugarcane bagasse are 3.82% ash,
22.09% lignin, 37.65% cellulose, 27.97% pentosan,
3.01% silica, and 3.3% reduction sugar. Basically,
fiber of sugarcane bagasse consists of cellulose,
pentosan, and lignin. Composition of each
component is vary depend on the variety of cane
(Mubin & Ratnanto, 2005) Sugarcane bagasse also
contains polysaccharides which can be converted
into many industry production. One of
polysaccharides components in sugarcane bagasse is
pentosan (20-27%).
With high concentration, pentosan from
sugarcane bagasse can be converted into furfural.
Furfural has aldehyde carbonyl functional group
which can be transformed into its derivatives like
alcoholic furfuryl, furan, etc. Schiff Base is one of
organic compounds which contain imine (-HC=N-)
which can be synthesized from condensation of
carbonyl group like aldehyde or ketone with primary
amine (Cinerman et al., 1997). Schiff Base is one of
organic compound with many uses. It can be use as
pigment and dye, catalyst, intermediate in organic
synthesize, and polymer stabilizer (Dhar & Thappo,
1982). Some of research have found that Schiff Base
can be used as corrotion inhibitor for metal which
spontaneously form a layer to protect materials and
friendly environmentally (Li et al., 1999). Previous
researchers have synthesized Schiff Base from
cynnamaldehyde with 2-aminophenol as corrotion
inhibitor for iron in HCl 0.5 N as media with
inhibitor efficiency 92% (Qasim, 2011). Schiff Base
from condensation of cynnamaldehyde as the source
of carbonyl with ethylenediamine as the source of
amine in concentration 7000 ppm for zinc in HCl
0.1N as media with inhibitor efficiency 90.17%
(Ginting et al., 2016). Corrotion is a decrease quality
of metal because electrochemical reaction with
environment. (Trethewey and Chamberlain,
1991).Corrotion is a big problem for metal-formed
material thing like car, bridge, ship machine, etc
(Riegher, 1992). Metals can be broken and lost its
function because of corrotion. Corrotion in many
things can not be prevented but the rate of corrotion
can be inhibited (Callister, 1991). Based on the
descrioption, we interest to synthesize Schiff Base
from furfural from sugarcane bagasse isolation
wirhethylenediamine. The result obtained is
expected has corrotion inhibitor activity and can be
tested in rusty zinc with HCl 0.1 M as media.
Ginting, M., Bulolo, D., Sihotang, H. and Masmur, I.
Corrosion Inhibitors Activity of Schiff Base from Condensation of Ethylenediamine with Furfural from Sugarcane Bagasse.
DOI: 10.5220/0010136500002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 71-77
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
71
2 RESEARCH METHODOLOGY
2.1 Materials and Methods
Used equipments in this research are : condenser,
hotplate stirrer, thermometer, vacuum distillation
tools, analytical balance, UV-Vis
Spectrophotometer, FT-IR Spectrophotometer,
desiccators, and glasswares. Then used materials in
this research are sulfuric acid (p.a), ethanol (p.a),
ethylenediamine (p.a), furfural, zinc plate, aquadest,
sugarcane bagasse, sodium chloride (p.a), and
hidrocloric acid (p.a).
2.2 Isolation of Furfural from
Sugarcane Bagasse
Put 100 grams of dried sugarcane bagasse in one-
neck flask 250 ml then add 100 grams of NaCl and
H
2
SO
4
10% until sink and stir until homogeneous.
Arrange distillation tools and reflux the mixture for
5 hours in 106
o
C. Furfural and water will be
condensed to distillation flask then put the drop of
liquid into Erlenmeyer which contain chloroform.
Furfural will be soluted in chloroform and water will
be separated in two layers (top layer is water and
bottom layer is furfural/chloroform). The mixture of
water and chloform is separated by dropping funnel.
Then, add 1 gram Na
2
SO
4
anhidrous to chloroform
layer to separate water and filter it. The filtrate then
distillate in 61
o
C-65
o
C to evaporate chloroform.
Furfural residue is purified by vacuum distillation
where furfural is in destilate form in 130
o
C/8
mmHg. Identify the colour by using aniline acetate
(1:1 v/v) as solvent, then analysed by UV-Vis
Spectrophotometer and FT-IR Spectrophotometer.
2.3 Synthesize Schiff Base from
Condensation of Furfural with
Ethylenediamine
Put 9.6 grams (0.1 mol) of furfural in two neck flask
250 ml then soluted with 25 ml ethanol absolute.
Arrange reflux tools with magnetic bar,
thermometer, and water trap. Put 2,4 grams (0.04
mol) of ethylenediamine which soluted with 25 ml
ethanol absolute and drop slowly by dropping funnel
and stir with reflux condition for 5 hours. The
solution then evaporate by rotaryevaporator, then
excess furfural is evaporated by vacuum distillation.
Then, dry residue from evaporation in desiccators
and weight on analytical balance and analyse by FT-
IR Spectroscopyparagraphs.
2.4 Determination of Inhibitor
Efficiency
Soaking solution for zinc plate is taken from
inhibitor solution 1000 ppm, put 150 ml in a
glassware. Soak sanded zinc 5 cm x 1 cm in the
solution for 24 hours. Take the plate from media,
wash carefully, then dry for 5 minutes and weigh the
final weight. Calculate inhibitor efficiency by using
this formula (Chitra et al., 2010):
%EI
W
W
W
100%
Where
EI = Inhibitor Efficiency
W
0
= The lost of weigh without inhibitor use
W
1 =
Thelost of weigh with inhibitor use
As comparation (control) use solution without
inhibitor. With the same procedure, do the same
thing with corrotion inhibitor variation 3000 ppm,
5000 ppm, and 7000 ppm in 48, 72, 96, and 120
hours. Do the same procedure for furfural,
ethylenediamine, and Schiff Base.
3 RESULT AND DISCUSSION
3.1 Isolation of Furfural
Isolation of furfural is by hydrolysis pentosan from
sugarcane bagasse to pentose by HCl and
dehydration pentose by sulfuric acid to form
furfural. Qualitative analysis for furfural by use
aniline acetate shows brick red colour. 100 g
sugarcane bagasse yields 5.2 g (5.2%) furfural. HCl
is produced by reaction between NaCl with excess
H
2
SO
4
then excess H
2
SO
4
is used as dehydrator to
form furfural (figure 1).
Figure 1: Furfural Formation Reaction.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
72
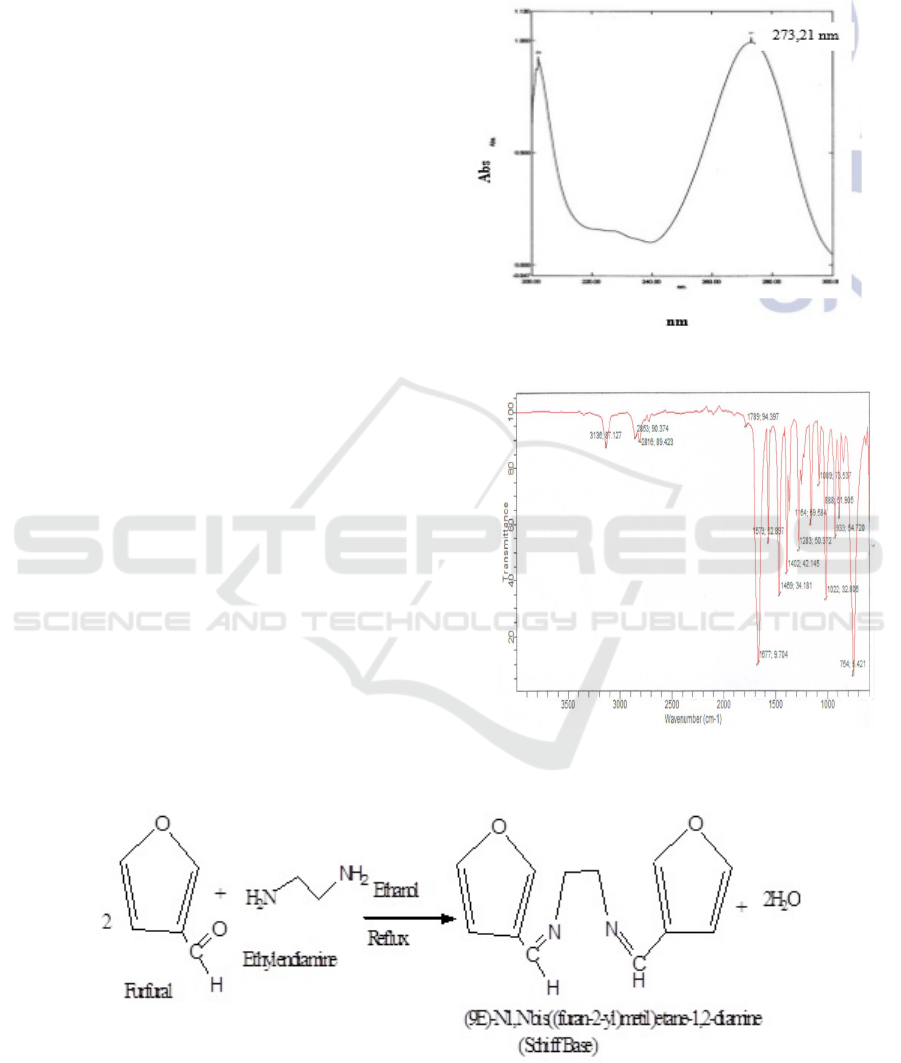
UV-Vis spectroscopy analysis shows λmax in 273.1
nm which indicate furfural have conjugated diene
bond (picture 2). FT-IR Spectroscopy result for
furfural shows spectrum peak in 3100 cm
-1
for
stretching aromatic C-H, 2900-2800 cm
-1
for
stretching C-H aldehyde, 1740-1720 cm
-1
for
stretching C=O, 1600-1475 cm
-1
for aromatic C=C,
1300-1000 cm
-1
for stretching C-O-C, and 1500-
1300 cm
-1
for bending C-H aldehyde (figure 3).
3.2 Synthesize of Schiff Base (9E)-
N
1
,Nbis((furan-2-yl)metil)etane-1,2-
diamine from Condensation of
Furfural with Ethylenediamine
Schiff Base is produced by condensation between
furfural and ethylenediamine as the Schiff Base is
produced by condensation between furfural and
ethylenediamine as the source of primary amine with
ethanol as the solvent reflux for 5 hours (picture 4).
The product then purified by vacuum distillation.
Condensation of 9.6 g (0.1 mol) furfural with 2.4 g
(0.04 mol) ethylenediamine yields 8.64 g (70.55%)
Schiff Base. Spectroscopy analysis for Schiff Base is
indicated by vibration peak in 1647 cm
-1
as –HC=N
3.3 Corrotion Inhibitor Efficiency
Calculation
Inhibitor efficiency is tested by soaking zinc plate in
corrotion media solvent HCl 0.1 N by using furfural,
ethylenediamine, Schiff Base as inhibitor and media
without inhibitor. Time variation are 24 hours, 48
hours, 72 hours, 96 hours, and 120 hours. Variation
of inhibitor concentration are 1000 ppm, 3000 ppm,
5000 ppm, and 7000 ppm. Average inhibitor
efficiency value can be look in table 1, 2, 3, and 4
source of primary amine with ethanol as the solvent
reflux for 5 hours (picture 4). The product
Figure 2: UV-Vis Spectrum for Isolated Furfural.
Figure 3: FT-IR Spectrum for Furfural.
Figure 4. Formation and reaction of Schiff Base by condensation furfural with ethylenediamine.
Corrosion Inhibitors Activity of Schiff Base from Condensation of Ethylenediamine with Furfural from Sugarcane Bagasse
73
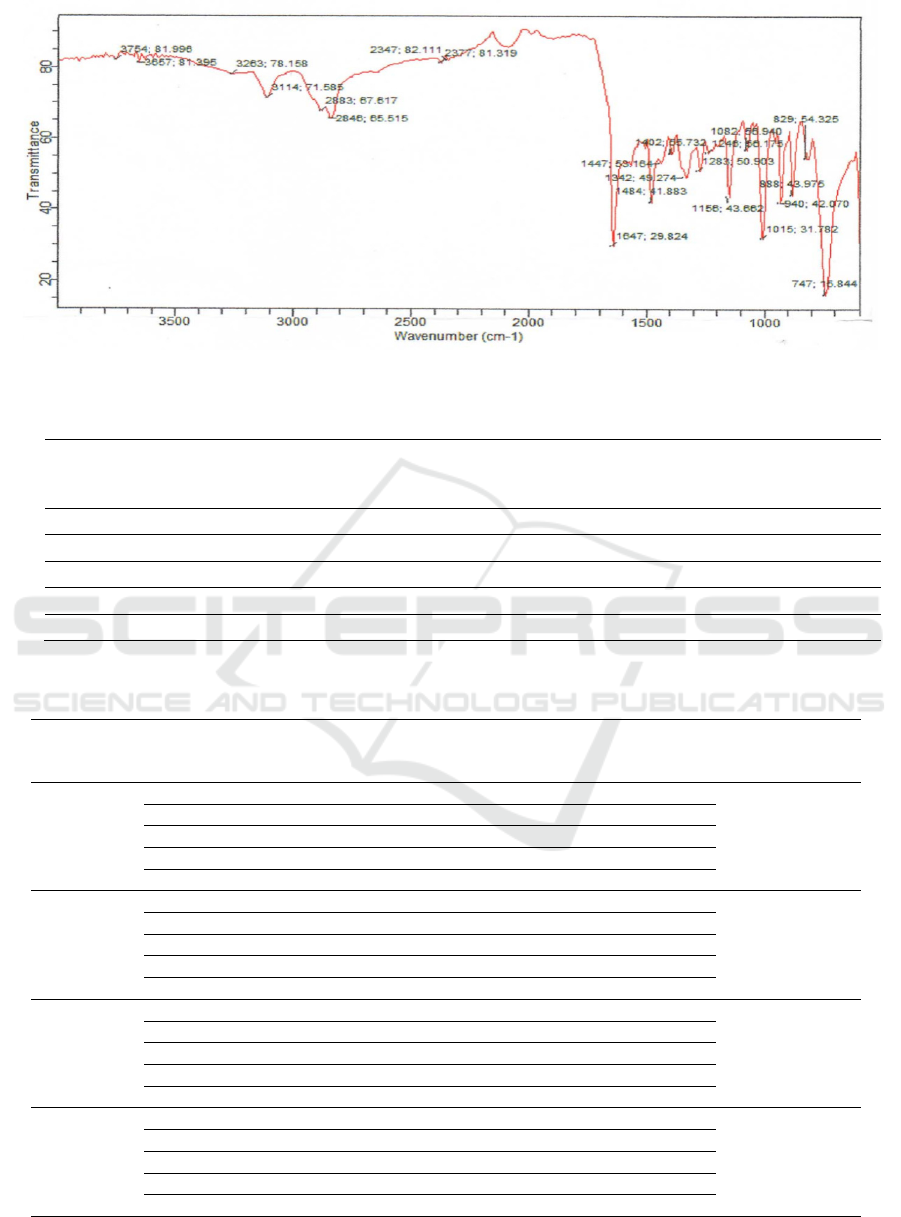
Figure 5: FT-IR Spectrum of Schiff Base (9E) )-N
1
,N
2
-bis((furan-2-yl)methyl)etane-1,2-diamine.
Table 1: Result of Zinc Plate Soaking without inhibitor in Corrosive Media Solution HCl 0.1 N.
Inhibitor
Concentration
(ppm)
Soaking
Time
(hours)
Zinc
Initial
Weight (g)
Zinc
Final
Weight (g)
Lost of
Weight
(g)
Inhibitor
Efficiency
(%)
0 24 2.9909 2.8811 0.1098
0 48 3.3514 3.2309 0.1205
0 72 3.2925 3.1623 0.1302
0 96 3.2750 3.1047 0.1703
0 120 3.2957 3.0613 0.2344
Table 2: Result of Zinc Plate Soaking with Schiff Base (9E)-N
1
,N
2
-bis((furan-2-yl)methyl)etane-1,2-diamine as inhibitor in
Corrosive Media Solution HCl 0.1 N.
Inhibitor
Concentratio
n
(ppm)
Soaking
Time
(hours)
Zinc Initial
Weight (g)
Zinc Final
Weight (g)
Lost of
Weight (g)
Inhibitor
Efficiency
(%)
Average Inhibito
r
Efficiency (%)
1000
24 3.503
1
3.4522 0.0509 53.64
49.48
48 3.422
1
3.3644 0.0577 7.38
72 3.334
2
3.2548 0.0794 39.01
96 3.302
1
3.2518 0.0503 70.46
120 3.235
8
3.1817 0.0541 76.91
3000
24 3.213
3
3.2133 0.0381 68.38
59.71
48 3.110
6
3.0612 0.0494 55.00
72 3.124
4
3.0580 0.0664 61.00
96 3.752
5
3.6832 0.0693 70.43
120 2.410
5
2.3373 0.0732 43.77
5000
24 3.277
7
3.2540 0.0237 71.41
77.88
48 3.514
9
3.4819 0.033 72.61
72 3.516
7
3.4827 0.034 73.88
96 3.386
0
3.3544 0.0316 81.44
120 3.512
5
3.4729 0.0396 83.10
7000
24 3.253
0
3.2310 0.022 79.96
82.20
48 3.641
4
3.6119 0.0295 75.51
72 3.112
1
3.0912 0.0209 83.94
96 3.556
6
3.5214 0.0352 79.33
120 3.134
7
3.1166 0.0181 92.27
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
74

Table 3: Result of Zinc Plate Soaking with Furfural as Inhibitor in Corrosive Media Solution HCl0.1 N.
Inhibitor
Concentration
(ppm)
Soaking
Time
(hours)
Zinc
Initial
Weight(g)
Zinc
Final
Weight (g)
Lost of
Weight
(g)
Inhibitor
Efficiency
(%)
Average
Inhibitor
Efficiency
(%)
1000
24 2.3305 2.2880 0.0425 61.29
45.60
48 2.1419 2.0972 0.0447 60,90
72 3,5105 3.4533 0.0572 56.06
96 3.6501 3.5012 0.1489 12.56
120 3.4551 3.3033 0.1518 35.23
3000
24 3.0941 3.0357 0.0584 46.81
51.10
48 3.2933 3.2139 0.0794 34.10
72 3.3144 3.2519 0.0625 51.99
96 3.9606 2.8909 0.0697 59.07
120 3.5411 3.4557 0.0854 63.56
5000
24 3.1454 3.0964 0.049 55.37
62.89
48 3.1520 3.1012 0.0508 57.84
72 3.1358 3.0822 0.0536 58.83
96 3.1314 3.0757 0.0557 67.29
120 3.2515 3.1932 0.0583 75.12
7000
24 3.2219 3.1844 0.0375 65.84
69.06
48 3.2116 3.1737 0.0379 68.54
72 3.0415 3.3572 0.0443 65.97
96 3.1725 3.1240 0.0485 71.52
120 3.1443 3.0821 0.0622 73.46
Table 4: Result of Zinc Plate Soaking with Ethylenediamine as Inhibitor in Corrosive Media Solution HCl 0.1 N.
Inhibitor
Concentration
(ppm)
Soaking
Time
(hours)
Zinc
Initial
Weight (g)
Zinc
Final
Weight (g)
Lost of
Weight
(g)
Inhibitor
Efficiency
(%)
Average
InhibitorEffic
iency
(%)
1000
24 3.2418 3.1862 0.0556 49.36
40.35
48 3.1776 3.1082 0.0694 42.40
72 3.5145 3.4373 0.0772 40.70
96 3.3858 3.3010 0.0848 50.20
120 3.3211 3.1315 0.1896 19.11
3000
24 3.3116 3.2486 0.0630 42.62
43.88
48 3.3355 3.2551 0.0804 33.27
72 3.3845 3.3143 0.0702 46.08
96 3.5643 3.4665 0.0978 42.57
120 3.3442 3.2385 0.1057 54.90
5000
24 3.5110 3.4312 0.0789 28.14
49.02
48 3.3651 3.3120 0.0531 55.93
72 3.2741 3.2114 0.0627 51.84
96 3.3315 3.2418 0.0897 47.32
120 3.1308 3.0415 0.0893 61.90
7000
24 3.2182 3.1887 0.0295 73.13
53.67
48 3.3888 3.2080 0.0808 32.94
72 3.4141 3.3215 0.0926 28.87
96 3.2388 3.1811 0.0577 66.11
120 3.2146 3.1380 0.0766 67.32
Corrosion Inhibitors Activity of Schiff Base from Condensation of Ethylenediamine with Furfural from Sugarcane Bagasse
75
3.4 Result of Determination Inhibitor
Efficiency
Determination of corrotion inhibitor efficiency is by
soaking zinc plate in HCl 0.1 N as media for 24, 48,
72, 96, and 120 hours with concentration variation
1000 ppm, 3000 ppm, 5000 ppm, and 7000 ppm.
Efficiency inhibitor is tested in zinc because it is an
active metal and always used in industry (Shah et al.,
2011). The metal will be reduced and oxidized.
3.5 The Effect of Soaking Time to the
Lost Weight of Zinc in Corrotion
Media HCl 0.1 N
The effect of soaking time to the corrotion increase
in HCl 0.1 N based on variation time 24, 48, 72, 96,
and 120 hours is very high. It proves that hidrocloric
acid solution is a corrosive media. The speed of
corrotion for zinc is parallel with the length of
soaking time (table 1).
3.6 The Effect of Inhibitor
Concentration to the Lost Weight
of Zinc in Corrosive Solution
Media HCl 0.1 N
The increase of inhibitor concentration is parallel
with the decrease of lost weight in zinc. It is because
adsorption of inhibitor molecules in the surface of
zinc platecan form shielding layer from free electron
in atoms like O, N, and phi bonding which limit O
2
diffusion in zinc surface.
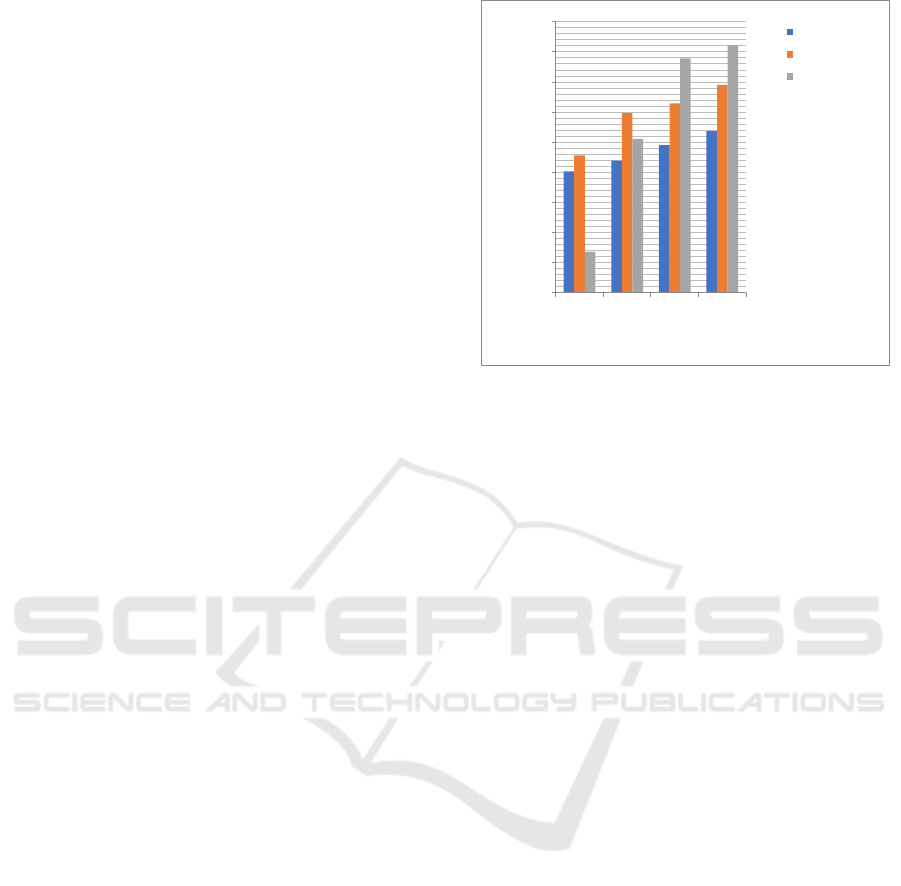
3.7 The Effect of Increasing Inhibitor
Concentration to Inhibitor
Efficiency in Zinc Plate with HCl
0.1 N as Corrotion Media
Inhibitor compounds in this research are furfural,
ethylenediamine, and Schiff Base. The compounds
are active as corrotion inhibitor. Inhibitor efficiency
is increase parallel with the increase of inhibitor
concentration in HCl 0.1 N as corrosive media.
Figure 7: Graph of The Effect of Inhibitor Concentration
to Inhibitor Efficiency.
4 CONCLUSSIONS AND
SUGGESTIONS
4.1 Conclusions
Based on the research result and data analysis, the
conclusions are:
1. Schiff Base can be synthesized by condensation
ethylenediamine with furfural from sugarcane
bagasse. The percentage of furfural from
sugarcane bagasse is 5.2%. The rendemen of
Schiff Base is 70.55%.
2. The result of Inhibitor efficiency test for zinc in
HCl 0. 1 N as corrosive media with concentration
7000 ppm are 82.20% for Schiff base, 53.67% for
ethylenediamine, and 69.06% for furfural.
4.2 Suggestions
The next researcher is expected to compare Schiff
base efficiency from furfural with another primary
amine source and the test of corrotion inhibitor in
metal with many corrosive media.
REFERENCES
Callister, W. D. (1991). Material Science and
Enggineering.An Introduction (2nd ed.).
Chitra, S., Parameswari, K., & Selvaraj, A. (2010).
Dianiline Schiff Base as Inhibitor of Mild Stell
Corrosion in Acid Media. Int. J. Electro Chemistry,
5(11), 1675–1697.
0
10
20
30
40
50
60
70
80
90
1000 3000 5000 7000
EficiencyInhibitor(%)
ConcentrationofInhibitor(ppm)
Ethylenediamine
Furfural
SchiffBase
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
76

Cinerman, Z., Galic, N., & Bosner, B. (1997). No Title.
..Anal.Chim.Acta., 343(1997), 145–151.
Dhar, D. N., & Thappo, C. L. (1982). Schiff Bases and
their Aplications. J. Sci. Ind. Res, 41(8), 501–506.
Ginting, M., Sihotang, H., & Manalu, R. (2016). Sintesis
Basa Schiff dari Hasil Kondensasi Sinama ldehida
Dengan Etilen diamina dan Fenil Hidrazin dan
Pemanfaatannya Sebagai Inhibitor Korosi Pada Logam
Seng. Prosiding Semirata, 1992–1998.
Hugot. (1986). Handbook of Cane Sugar Enginering (3rd
ed). Elsevier.
Li, S., Chen, S., Ma, H., Yu, R., & Liu, D. (1999).
Investigation on some Schiff bases as HCl
corrosioninhibitors for copper. Corrosion Science,
41(7), 1273–1287.
https://doi.org/https://doi.org/10.1016/S0010-
938X(98)00183-8
Mubin, A., & Ratnanto, F. (2005). Upaya Penurunan
Biaya Produksi dengan Memanfaatkan Ampas Tebu
sebagai Pengganti Bahan Penguat dalam Proses
Produksi Asbes Semen, Jurnal teknik Gelagar . 16(1),
10–19.
Qasim, M. (2011). Synthesis and characterization of new
Schiff bases and evaluation as Corrosion Inhibitor.
Riegher, H. . (1992). Electro chemistry. Chapman and
Hall Inc.
Shah, M. ., Patel, A. ., Mudaliar, G. ., & Shah, N. . (2011).
Schiff Bases of Triethylenetetramine as Corrosion
Inhibitors of Zinc in Hydrochloric Acid. Chemistry
Departement School of Sciences, Gujarat
University:Ahmedabad.
Corrosion Inhibitors Activity of Schiff Base from Condensation of Ethylenediamine with Furfural from Sugarcane Bagasse
77
