
A New Adsorbent Chitosan-based That Modified using
Epichlorohydrin and Diethylene Triamine for Treating Heavy Metal
(Cu
2+
, Zn
2+
and Fe
2+
)
Silitonga Regina Dewi
1
, Ginting Mimpin
1
and Kaban Jamaran
2
1
Postgraduated Chemistry Study Programme, Universitas Sumatera Utara, Medan, Indonesia
2
Department of Chemistry, Universitas Sumatera Utara , Medan, Indonesia
Keywords: Adsorption, Chitosan, Diethylen Triamin, Epichlorohydrin, Grafting, Heavy Metal
Abstract: In the current study, chitosan was modified using epichlorohydrin and diethylene triamine. Grafting
diethylene triamine chitosan is synthesized through alkylation of chitosan with epichlorohydrin and
followed by amination with diethylene triamine. The formation of chitosan grafted diethylene triamine was
confirmed using FT-IR spectrum which has a band at 1581 cm
-1
that assigned as C-N-C (secondary amine).
The morphological analysis using SEM showed the surface became smoother after grafting process. As
adsorbent, the chitosan grafted diethylene triamine had the adsorption capacity 72, 67, and 63 ppm for Cu
2+
,
Zn
2+
, and Fe
2+
, respectively.
1 INTRODUCTION
As a developing country, the government set the
industrial sector to be the first priority. The growth
of new industries gives many negative impacts to the
environment, in the form of solid and liquid waste
that quite dangerous for human (Cahayaningrum et
al., 2011). In the study that conducted by Laksono et
al. (2008), in the solid or liquid waste it will be
common to obtain the presence of heavy metal
waste, as the example in the painting industry, in the
liquid waste will be found the ion of Cu, Fe, Cr, Ni,
and Zn.
The current pattern of industrial activity alters
the natural flow of materials and introduces novel
chemicals into the environment (Faisal & Hasnain,
2004). The rate at which effluents are discharged
into the environment especially water bodies have
been on the increase as a result of urbanization.
Most of these effluents contain toxic substances
especially heavy metals. The presence of heavy
metals in the environment is of major concern
because of their toxicity, bio-accumulating tendency,
threat to human life and the environment (Horsfall &
Spiff, 2005; Igwe & Abia, 2003).
Several treatments have been developed to solve
this kind of issue, i.e. precipitation, ion exchange
resin, filtration, and adsorption (Tangio, 2013).
Adsorption technique is the quite effective method
because of the low operational cost, high efficiency
and easy method for regenerating (Juir et al., 2017).
This method can be done by utilizing natural
polymers (biopolymers) as an adsorbent with one of
them, namely chitosan (Schumul et al., 2001).
Chitosan is the result of deacetylation of chitin
that commonly found in crabs, shrimp and squid
(Bhatnaga & Sillanpaa, 2009). Chitosan consist of
glucosamine unit and N-acetyl glucosamine unit
(Ngah & Fatinathan, 2010). Chitosan as an
adsorbent has highest adsorption capacity when
compared activated carbon, peat, biomass,
agricultural solid waste, industrial by products,
silica, zeolite and clay (Crini & Badot, 2008).
Abudantly the availability and ease of modification
process make chitosan become one of the
ingredients adsorbents are widely use in process
adsorption (Ngah & Fatinathan, 2010).
Adsorption is the ability of the adsorbate to
adhere or attach to the adsorbent. It is a well
established separation technique to remove dilute
pollutants as well as to recover valuable products
from aqueous streams. In the conventional
adsorption process, the particle size of the adsorbent
is restricted because of hydrodynamic phenomena
such as pressure drop.
Regina Dewi, S., Mimpin, G. and Jamaran, K.
A New Adsorbent Chitosan-based That Modified using Epichlorohydrin and Diethylene Triamine for Treating Heavy Metal (Cu2+, Zn2+ and Fe2+).
DOI: 10.5220/0010133400002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 65-70
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
65

Solid surfaces that come into contact with a
solution tend to collect layers of solute molecules on
their surface due to an imbalance of forces on the
surface. Chemical adsorption results in the formation
of a monomolecular layer of adsorbate on the
surface through the forces of the residual valence of
the molecules on the surface. Physical adsorption
results from molecular condensation in the
capillaries of the solids. In general, elements with
greater molecular weight will be more easily
adsorbed. There is a rapid formation of an
equilibrium interface concentration, followed by
slow diffusion into carbon particles. The overall
adsorption rate is controlled by the diffusion rate of
solute molecules in the capillary pores of carbon
particles (Malkoc et al., 2006).
The use of the neat chitosan as adsorbent is not
effective due to its high solubility in the acid
medium, especially in acetic acid solution, HNO
3
,
HCl, etc. To resolve the issue, a modification
technique is needed to improve the performance of
chitosan, this modification can give several
advantages, i.e. could be use for several cycles and
enhance the stability of chitosan. If an amine
compound added to the chitosan’s structure, at the
end the number of amine group in chitosan will
increase. As the impact, it will improve the
adsorption capacity due to the new bond, also the
selectivity and stability of adsorption.
This study is involved with the introduction of
diethylene triamine into chitosan backbone through
the reaction of an intermediate of epoxy activated
chitosan and diethylene triamine. On the other hand,
several studies have indicated that amino groups in
chitosan are the main sites for the adsorption. Yi et
al. (2006) synthesized chitosan diethylene triamin
(DETA) epoxy and compared the capacity of
crosslinked chitosan and diethylene triamin chitosan
epoxy in adsorbing Pd
2+
, Ag
+
, Ni
2+
, Cu
2+
, Cd
2+
and
Co
2+
. Yan et al. (2013) carried out the preparation
and adsorption properties of chitosan granules
modified with diethylene triamine for acid dye
adsorption. Juir et al. (2017) crosslinked
epichlorohydrin with chitosan to test the mechanical
properties and absorption as an adsorbent.
Based on the description above, in this study
synthesis of chitosan compound which has been
grafting with diethylene triamin (DETA) which
occurs through alkylation with epichlorohydrin and
amination with diethylene triamin (DETA) and
adsorption testing of Cu
2+
, Fe
2+
and Zn
2+
metal ions.
2 MATERIAL AND METHODS
2.1 Material
Chitosan was purchased from Merck (DDA 80%).
Acetic acid glacial, sodium hydroxide, methanol,
ethanol, acetone, ether, nitric acid, copper sulfate
pentahydrate, iron sulfate heptahydrate, and zinc
sulfate heptahydrate were obtained from Merck.
Epichlorohydrin and
diethylene triamine were
purchased from TCI.
2.2 Characterization
The functional group of material was determined
using FT-IR (Shimadzu). The morphological surface
material was analyzed using SEM (JSM-35 C
Sumandju). The
presence on heavy metal in the
material after adsorption process was determine
using atomic absorption spectrometer (GF Perkin
Elmer).
2.3 Synthesis of Epoxy Chitosan
About 3 g of chitosan was suspended in 250 mL of
sodium hydroxide 0.4 M and 30 mL of
epichlorohydrin, the mixture was stirred for 5 h at
temperature of 50
o
C. The obtained residue was
washed using distillate water, acetone, and ether,
respectively. The residue was dried until the
constant weight was obtained
2.4 Chitosan Grafted Diethylene
Triamine
About 0.5 g of epoxy chitosan was suspended in 30
mL of sodium hydroxide 0.1 M and 0.5 g of
diethylene triamine, this mixture was stirred for 4 h
at temperature of 60
o
C. the obtained residue was
washed using distillate water, alcohol, and acetone,
respectively. The residue was dried until the
constant weight was obtained. The above procedure
was repeated for the other variation of diethylene
triamine (1 and 1.5 g).
2.5 Adsorption of Heavy Metal
Solution
The adsorption of heavy metal ion was performed
using the sulfate salt solution of each heavy metal,
i.e.CuSO
4
.5H
2
O, FeSO
4
.7H
2
O, ZnSO
4
.7H
2
O. About
0.01 g chitosan grafted diethylene triamine was
weighed and suspended in 50 mL of the heavy metal
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
66
solution (200 ppm). The mixture was stirred for 20
min at room temperature and then filtered. The
obtained filtrated was added with concentrated nitric
acid and the pH was adjusted into 3. The final
solution then was measured using AAS.
The capacity adsorption of modified chitosan can
be determined using the following equation:
𝑞
𝐶𝑜 𝐶
𝐶𝑜
100%
where, q was the percentage of capacity adsorption.
Co and C was the heavy metal ion concentration
before and after treatment (ppm). In Fig. 1 below
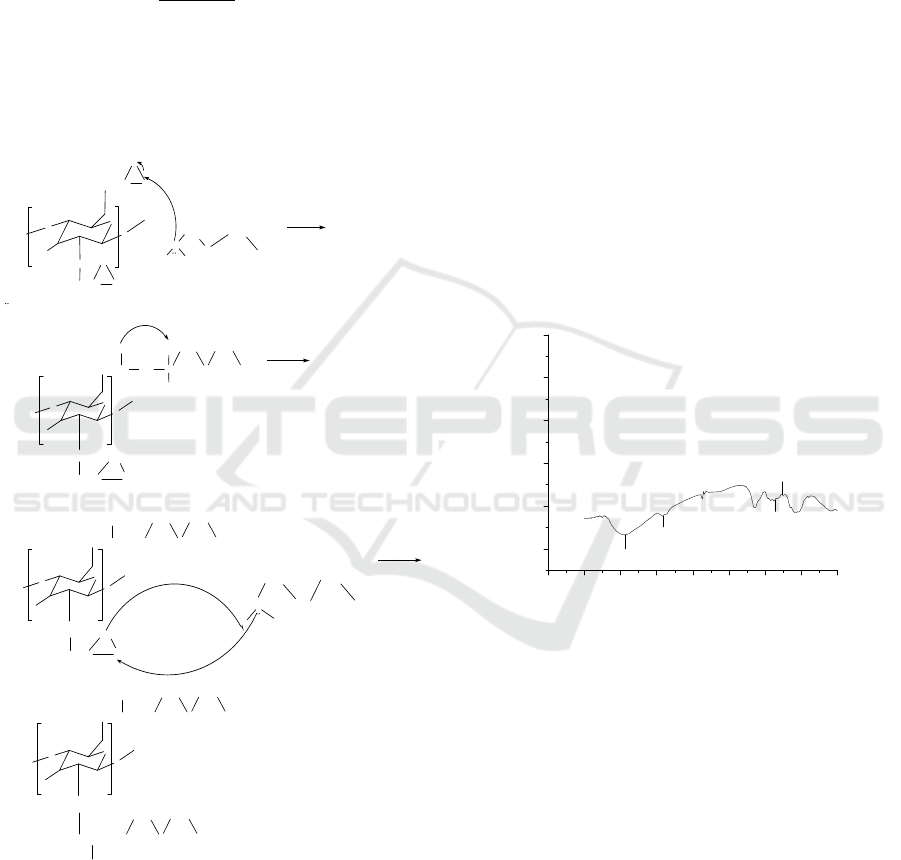
showed the proposed mechanism of the formation of
chitosan grafted diethylene triamine.
CH2CH CH
2
O
CH2CH CH
2
O
O
O
O
NH
HO
O
n
Epoksi Kitosan
+
N
CH
2
CH
2
H
H
N
H
CH
2
CH
2
NH
2
CH
2
CH
2
CH
2
CH
2
NH
2
NH
N
O
O
O
HO
O
n
CH2CH CH
2
O
H
H
NaOH
NaOH
NH
CH2CH
O
CH
2
CH
2
CH
2
CH
2
CH
2
NH
2
NH
HN
O
O
O
HO
O
n
CH
2
CHCH
2
OH
NH
CH2CH
O
CH
2
N
CH
2
CH
2
H
H
NH
CH
2
CH
2
NH
2
CH
2
CH
2
CH
2
CH
2
NH
2
NH
HN
O
O
O
HO
O
n
CH
2
CHCH
2
OH
NH
CH
2
CHCH
2
+
OH
CH
2
CH
2
CH
2
CH
2
NH
2
NH
HN
Figure 1: Proposed mechanism of the formation of
chitosan grafted diethylene triamine.
3 RESULT AND DISCUSSION
3.1 FT-IR
Chitosan grafted diethylene triamine was
synthesized using chitosan, epichlorohydrin, and
diethylene triamine. This synthesis was performed in
two steps, i.e. the formation of epoxy chitosan and
the grafting of diethylene triamine
.
In the first step, alkylation reacts with chitosan
with epichlorohydrin. Where the OH group changes
in chitosan to C-O-C epoxy. The FT-IR spectrum
(Figure 2) shows that the wave number 3448.72 cm
-1
shows the -OH and -NH
2
groups (Sastrohadmijojo,
2018). At wave number 2924.09 cm
-1
shows
stretching –CH (sp
3
) bonds (Hartomo & Purba,
1986). The amine C-N group appears at wave
number 1381.03 cm
-1
. For C-O-C epoxy groups
appear at wave number 1319 cm
-1
. At wave number
1072.42 cm
-1
is a C
1
-C
5
bond and is a β-1,4-
glycosidic bond (Pavia, 2001).
4500 4000 3500 3000 2500 2000 1500 1000 500
0
20
40
60
80
100
Transmitation
wavelength
OH, -NH
C-H (sp
3
)
C-N
C-O-C epoxy
Figure 2: FT-IR Result of Epoxy Chitosan.
In the second step, the formation of diethylene
triamin chitosan grafting through the epoxy chitosan
amination with diethylene triamin. The FT-IR
spectrum (Figure 3) shows the -OH and -NH
2
groups
that appear at the wave number 3425.58 cm
-1
(Sastrohadmijojo, 2018). At wave number 2924.09
cm
-1
indicates the presence of –CH (sp
3
) bonds
(Hartomo & Purba, 1986). The formation of C-N-C
groups in diethylene triamin chitosan grafting
compounds appears at wave number 1458.18 cm
-1
.
The amine C-N group appears at wave number
1381.03 cm
-1
. At wave number 1080.14 cm
-1
is a C
1
-
C
5
bond and is a β-1,4-glycosidic bond (Pavia,
2001).
A New Adsorbent Chitosan-based That Modified using Epichlorohydrin and Diethylene Triamine for Treating Heavy Metal (Cu2+, Zn2+
and Fe2+)
67
4500 4000 3500 3000 2500 2000 1500 1000 500
0
10
20
30
40
50
60
Transmitation
wavelength
OH,-NH
CH (sp
3
)
C-N
C-N-C
Figure 3: FT-IR spectrum of modified chitosan (chitosan
grafted DETA (with varian DETA).
3.2 Scanning Electron Microscopy
(SEM)
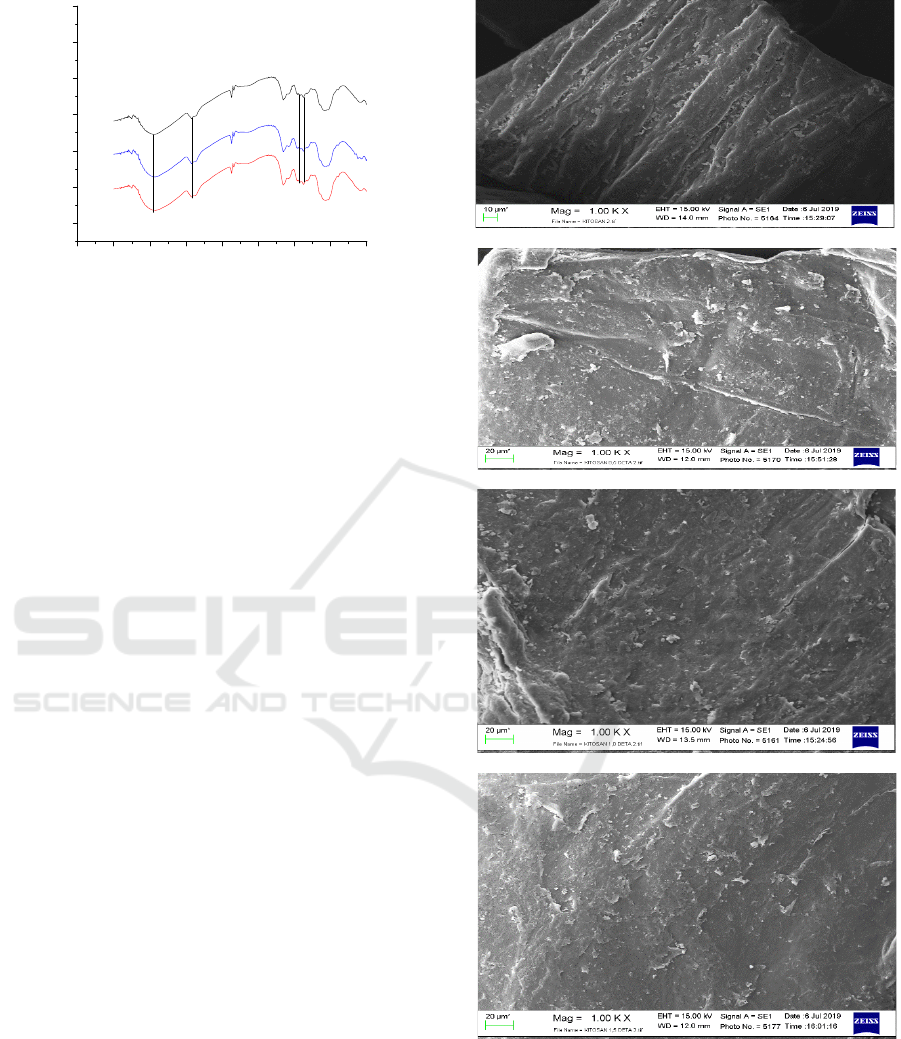
Fig. 4 showed the morphological surface of chitosan
and modified chitosan which has a significant
difference. The number of chitosan fibril on the
surface was decrease after the addition of
epichlorohydrin and diethylene triamine. The
increase of diethylene triamine caused the film’s
surface became smoother. The presence of tiny
particle and crust layer on the film’s surface can be
indicated as the successful of grafting process. The
change of surface morphology as the result of
chemical interaction between the hydroxyl and aine
of chitosan. With this change in the film’s
morphology could improve the adsorption
performance.
3.3 Adsorption of Heavy Metal
Adsorption performance of modified chitosan to
Cu
2+
, Zn
2+
dan Fe
2+
can be seen in Table 1 and Table
2. Chitosan grafted with 1.5 g diethylene triamine
showed the highest performance to adsorb Cu
2+
, with
adsorption capacity 72.0 ppm. It adsorbed 36% of
Cu
2+
that presence in the solution of Cu
2+
200 ppm.
The obtained of this result as the impact of the
modification of chitosan structure. This modification
improves the reactivity of chitosan due to the
presence of new amine group from diethylene
triamine in the modified chitosan. The increase of
amine group improves the ability of modified
chitosan to form a chelate with the heavy metal ion,
it means the adsorption capacity will enhance. The
lone pairs electron in nitrogen can be used to form a
complex with transition metal through coordination
bonding (Lerivrey et al., 1986).
(a)
(b)
(c)
(d)
Figure 4: SEM images of a) chitosan b) chitosan grafted
0.5 g diethylene triamine, c) chitosan grafted 1.0 g
diethylene triamine, d) chitosan grafted 1.5 g diethylene
triamine.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
68

Table 1: The adsorption capacity of modified chitosan.
Adsorbent
Adsorption capacity
(mg/L)
Cu
2+
Zn
2+
Fe
2+
Chitosan 25.7 27.0 37.2
Chitosan grafted 0.5 g
diethylene triamine
51.8 48.8 58.9
Chitosan grafted 1.0 g
diethylene triamine
64.3 52.1 62.4
Chitosan grafted 1.5 g
diethylene triamine
72.0 67.0 63.9
Table 2: Adsorp of modified chitosan.
Adsorbent
% Adsorb
Cu
2+
Zn
2+
Fe
2+
Chitosan 12.8 13.5 18.6
Chitosan grafted 0.5 g
diethylene triamine
25.9 24.4 29.5
Chitosan grafted 1.0 g
diethylene triamine
32.2 26.1 31.2
Chitosan grafted 1.5 g
diethylene triamine
36.0 33.5 32.0
Figure 5: Adsorption capacity curve of modified chitosan.
According to Fig. 5, the highest performance
from three kinds of modified chitosan is shown in
chitosan grafted with 1.5 g diethylene triamine,
especially for adsorbing Cu
2+
. This is caused by the
highest acidity level of Cu
2+
compared to Zn
2+
and
Fe
2+
, it caused Cu
2+
is easier to form complex with
chitosan. The ability of adsorbent to adsorb the
specific component is influenced by pH, related to
protonation or deprotonation of adsorbent’s active
site. The system’s pH will influence the charge on
the adsorbent surface, degree of ionization, and the
species that able to absorb in the process.
4 CONCLUSIONS
Chitosan grafted diethylene triamine was
synthesized through alkylation and amination using
epichlorohydrin and diethylene triamine,
respectively. The formation this modified chitosan
was confirmed from FT-IR spectrum with the
presence of C-N-C vibration at 1581 cm
-1
. The
morphological analysis showed the surface became
smoother after grafting process. The modified
chitosan has specific adsorption capacity for each
heavy metal ion, i.e. Cu
2+
= 72,0 ppm; Zn
2+
= 67.0
ppm dan Fe
2+
= 63.9 ppm.
ACKNOWLEDGEMENTS
The authors would like to thank to University of
North Sumatera, Medan for their support in use of
laboratories and for my supervisor who has provided
the benefits of guidance and advice in conducting
this research.
REFERENCES
Bhatnaga, A., & Sillanpaa, M. (2009). Applications of
chitin- and chitosan-derivatives for the detoxification
of water and wastewater. Adv Colloid Interfac, 152,
26–38.
Cahayaningrum, S. E., Narsito, N., Santoso, S. J., &
Agustini, R. (2011). Adsorption of Mg (II) ion from
aqueous solution on Chitosan bends and chitosan
powder. Journal of Coustal Development, 13(3), 179–
184.
Crini, G., & Badot, P.-M. (2008). Application of chitosan,
a natural aminopolysaccharide, for dye removal from
aqueous solutions by adsorption processes using batch
studies: A review of recent literature. Progress in
Polymer Science, 33(4), 399–447.
https://doi.org/https://doi.org/10.1016/j.progpolymsci.
2007.11.001
Faisal, M., & Hasnain, S. (2004). Microbia conversion of
Cr(vi) into Cr(iii) in industrial effluent. African J.
Biotechnol., 3(11), 610–617.
Hartomo, A. J., & Purba, A. V. (1986). Penyidikan
spektrometrik senyawa organik. Erlangga.
Horsfall, M. J., & Spiff, A. I. (2005). Effects of
temperature on the sorption of ob2+ and cd2+ from
aqueous solution by caladium bicolor (wild cocoyam)
0,0%
10,0%
20,0%
30,0%
40,0%
0246
%Adsoprtion
AdsorptionCapacity
Cu2+
Zn2+
Fe2+
A New Adsorbent Chitosan-based That Modified using Epichlorohydrin and Diethylene Triamine for Treating Heavy Metal (Cu2+, Zn2+
and Fe2+)
69

biomass. Electronic Journal of Biotechnology, 8(2).
Igwe, J. C., & Abia, A. A. (2003). Maize Cob and Husk as
Adsorbents for removal of Cd, Pb and Zn ions from
wastewater. The Physical Sci., 2, 83–94.
Juir, N., Rahmi, & Marlina. (2017). Pengaruh penambahan
epiklorohidrin terhadap sifat mekanik dan daya serap
film khitosan sebagai adsorben. Jurnal Rekayasa
Kimia Lingkungan, 12(1), 31–36.
https://doi.org/https://doi.org/10.23955/rkl.v12i1.5094
Laksono, W. L., Projosantoso, A. K., & Ihsan, J. (2008).
Adsorpsi kitosan terhadap ion logam Ni (II) dan Mn
(II) pada berbagai pH. Penelitian Saintek, 13, 95–109.
Lerivrey, J., Dubois, B., Decock, P., Micera, G.,
Urbanska, J., & Kozłowski, H. (1986). Formation of
D-glucosamine complexes with Cu(II), Ni(II) and
Co(II) ions. Inorganica Chimica Acta, 125(4), 187–
190.
Malkoc, E., Nuhoglu, Y., & Abali, Y. (2006). Cr (VI)
adsorption by waste acorn of Quercus ithaburensis in
fixed beds: Prediction of Breaktrough curves.
Chemical Engineering Journal, 119, 61–68.
Ngah, W. S. W., & Fatinathan, S. (2010). Adsorption
characterization of Pb(II) and Cu(II) ions onto
chitosan-tripolyphosphate beads: kinetic, equilibrium
and thermodynamic studies. Journal of Environmental
Management, 91(4), 958–969.
Pavia, L. K. (2001). Introduction to Spectroscopy A guide
for students of organic chemistry (Third).
Sastrohadmijojo, H. (2018). Dasar-dasar spektroskopi.
UGM Press.
Schumul, R., Krieg, H. M., & Keizer, K. (2001).
Adsorption of Cu (II) and Cr (IV) by chitosan.
Kinetics and Equilibirium Studies Water, 27(1), 1–8.
Tangio, J. S. (2013). Adsorpsi logam Pb (Timbal) dengan
menggunakan biomassa enceng gondok (Eichhornia
crassipes). Jurnal Entropi, 8(1), 500–506.
Yan, Y., Xiang, B., Li, Y., & Jia, Q. (2013). Preparation
and adsorption properties of diethylenetriamine‐
modified chitosan beads for acid dyes. Journal of
Applied Polymer Science, 130(6), 4090–4098.
https://doi.org/https://doi.org/10.1002/app.39691
Yi, Y., Wang, Y., & Ye, F. (2006). Synthesis and
properties of diethylene triamine derivative of
chitosan. Colloids and Surfaces A: Physicochemical
and Engineering Aspects, 277(1–3), 69–74.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
70
