
The Effect of Inoculum Volume on Bioethanol Production from Saba
Banana Hump (Musa Paradisiacal. L) Starch by Zymomonas Mobilis
using Immobilization Technique
Rifqi Syahbana
1
, Emma Zaidar Nasution
2*
, Rumondang Bulan Nasution
2
1
Postgraduate Chemistry Study Programme, Universitas Sumatera Utara, Jl. Bioteknologi No. 1, Medan, Indonesia
2
Department of Chemistry, Universitas Sumatera Utara, Jl. Bioteknologi No. 1, Medan, Indonesia
Keywords: Bioethanol, Immobilization, Inoculum volume, Saba Banana Hump, Zymomonas Mobilis.
Abstract: Bioethanol is a liquid produced from the sugar fermentation process from carbohydrate sources by using the
microorganism. In present study, Saba banana hump (Musa paradisiacal. L) sample was isolated by
precipitating the starch with water for 12 hours. Furthermore, the starch was hydrolyzed using HCl 25%, and
14.01% of glucose solution was obtained. The starch obtained was tested using Fourier-transform infrared
spectroscopy. Then the sample was fermented using Zymomonas mobilis microorganism immobilized by Ca-
Alginate 4% with variation of inoculums 5%, 10%, 15% (v/v). Bioethanol from fermentation process was
tested using gas chromatography (GC). The highest bioethanol content is 79.01% and the highest productivity
that obtain was 2.66 g/L.Hours.
1 INTRODUCTION
Banana hump is the bottom of a banana plant that has
characteristics that are not too hard, banana hump
waste has a starch composition of approximately
70%, 20% water, the rest is protein and vitamins. For
the banana hump itself has not been used optimally,
where the banana weevil waste is sometimes only as
animal feed or left alone to rot. The carbohydrate
content of banana hump is very potential as a source
of bioethanol, so that it can increase the use value of
the banana weevil and can optimize the utilization of
the banana hump. As for one type of carbohydrate
that is in the banana weevil is starch.
Starch is a carbohydrate polymer consisting of
glucose monomers with the molecular formula
(C
6
H
10
O
5
)
n
. Starch is known as biocompatible,
biodegradable, non-toxic, environmentally friendly
and inexpensive natural polysaccharides (Atwell,
W.A., Hood, L., Lineback, D., Varriano-Morston, E.,
Zobel, 1988; Rodrigues and Emeje, 2012). Starch can
be a source of glucose in the fermentation process that
produces bioethanol by hydrolyzing starch with a
dilute acid solution or enzyme.
Bioethanol in the realm of renewable energy is
one source of energy that continues to be developed.
Bioethanol is the center of attention because this
compound can be used as fuel (Setiadji et al., 2017).
The raw materials for the bioethanol production
process are classified into three groups, namely sugar,
starch and cellulose. Sources of sugar derived from
cane sugar, beet sugar, molasses and fruits can be
directly converted to ethanol. Sources of starchy
ingredients such as corn, cassava, potatoes and plant
roots must first be hydrolyzed into sugar. Sources of
cellulose derived from wood, agricultural waste, pulp
and paper mill waste as a whole must be converted to
sugar with the help of mineral acids (Lin and Tanaka,
2006).
Z. mobilis is a gram-negative, stem-shaped
bacterium, can be found in sugar-rich sap plants, has
a length of 2-6 μm and a width of 1-1.4 μm, the
optimum temperature for growth is 25-30
o
C, and is
anaerobic. Z. mobilis can ferment glucose, fructose,
but cannot ferment silosa (Gunasekaran and Chandra
Raj, 1999; Geeta, 2007). Zymomonas mobilis
bacteria can survive on glucose level
Immobilization is the process of wrapping
(coating) a core material, in this case bacteria as a
core material by using its viability and protecting it
from damage due to environmental conditions that are
not possible (Wu, Roe and Gimino, 2000). (Pacifico,
Wu and Fraley, 2001) states that for sensitive
components such as microorganisms, it can be
Syahbana, R., Zaidar Nasution, E. and Bulan Nasution, R.
The Effect of Inoculum Volume on Bioethanol Production from Saba Banana Hump (Musa Paradisiacal. L) Starch by Zymomonas Mobilis using Immobilization Technique.
DOI: 10.5220/0010133200002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 53-58
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
53

immobilized to increase viability of shelf life.
Common ingredients used for immobilization are
various types of polysaccharides and proteins such as
starch, alginate, arabic gum, gelatin, carrageenan,
albumin, and casein.
Research has been carried out on immobilization
of zymomonas mobilis cells and saccharomyces
cerevisiae to increase the production of bioethanol
from sugar from the hydrolysis of sugarcane bagasse
waste. The results showed ethanol levels increased
using cells immobilized by the addition of sugarcane
bagasse to the modification of the calcium alginate
matrix, where the efficiency of fermentation in
zymomonas mobilis increased by 1.8 times while in
S. cerevisiae by 1.6 times (Kusumaningati, Nurhatika
and Muhibuddin, 2013).
The purpose of this study was to determine the
levels of bioethanol produced from zymomonas
mobilis fermentation with immobilization
techniques.
2 METHODS
2.1 Isolation of Starch from Kepok
Banana Weevil
Kepok banana weevil that is obtained is then peeled
and cleaned with clear water, then cut into cubes and
then put into a bucket and soaked with 0.2% Na
2
S
2
O
5
for 12 hours. Furthermore, the banana weevil is dried
in the sun, then blended until smooth and then filtered
using an 80 mesh sieve. Then dissolved with
Aquadest and deposited for 24 hours. Furthermore,
starch is separated from the solution and roasted at a
temperature of 45
o
C for 24 hours.
2.2 Starch Hydrolysis
The starch obtained was weighed as much as 20 g
then put into a 250 ml glass beaker, added as much as
100 ml 25% HCl and covered with alumanium foil.
Then it is heated at 80
o
C while stirring for 30 minutes.
After being cooled, the hydrolyzate was adjusted to
neutral pH using NaOH 0.7%.
2.3 Making YEPD (Yeast Extract
Pepton Dextrose) Media
The making of YEPD media is by dissolving 4 g
Yeast extract, 2 g KH
2
PO
4(s)
, 3 g (NH
4
)
2
SO
4(s)
, 1 g
MgSO
4
.7H
2
O
(s)
, 3.6 g Pepton and 2% Bacto agar with
1000 ml Aquadest. Then heated on a hotplate until it
is clear yellow.
2.4 Making Cell Immobilization
Nutrient media mixed with 20 gram (NH
4
) 2SO
4
18
gram starch, 10 gram NaHPO
4
, 5 gram KH
2
PO4, 5
gram MgSO
4
.7H
2
O and 1 gram yeast extract added to
culture. Put in the shaker incubator for 24 hours. Then
mixed 50 ml of nutrient media with 50 ml of 4%
alginate solution. Next 100 ml of Alginate-cell
mixture was added into 1000 ml of 2% CaCl
2
solution
until the solution was in the form of solids and
allowed to stand to harden for 30 minutes.
Subsequently washed solids with 0.85% NaCl and
incubated into the production medium with shaking
for 24 hours. Then the solid is stored in the yeast
extract at 4
o
C until the cell is used.
2.5 Hydrolyzed Fermentation using
Inoculum Z. Mobilis
Fermentation using inoculum Z. Mobilis by
dissolving 4 g Yeast extract, 2 g KH
2
PO
4(s)
, 3 g (NH
4
)
2SO
4
(s), 1 g MgSO
4
.7H
2
O
(s)
, 3.6 g Pepton and 2%
agar Bacto with 1000 ml of hydrolyzate. Then
sterilized using an autoclave for 2 hours at 121
o
C.
After the cold hydrolyzate was added inoculum Z.
Mobilis were 5%, 10%, 15% with the amount of
bacteria 9.0 x 10
8
(McFarland 3). Then it is tightly
closed using alumanium foil and plastic wrap and put
in a shaker incubator for 21 hours at 30
o
C while the
dishaker is at 100 rpm.
2.6 Separation of Bioethanol from
Fermentation Solutions
500 ml fermentation solution was put into 1000 ml
rotav flask then CaO was added to the fermentation
solution at a ratio of 1:2 (g / ml) and then in the rotary
evaporator at 78
o
C for 1 hour. Then the ethanol
qualitative test on the distillate obtained.
2.7 Quantitative Analysis of Bioethanol
using GC
Fermentation solution as much 500 ml was put into a
1000 ml rotav flask then CaO was added to the
fermentation solution at a ratio of 1:2 (g / ml) and then
in the rotary evaporator at 78
o
C for 1 hour. Then the
ethanol qualitative test on the distillate obtained.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
54

3 RESULTS AND DISCUSSION
3.1 Isolation of Starch from Saba
Banana Hump
The results of isolation of starch from Saba Banana
Hump by stopping the browning process using 0.2%
Na
2
S
2
O for 12 hours, so that the squeeze process will
produce a white milk solution and deposited for 24
hours. From 1000 grams of saba banana hump
obtained 157 grams of starch banana weights that are
milky white. The results of Saba Banana Hump starch
can be seen in Figure 1
Figure 1: Results of Isolation of Starch from Saba
Banana Hump.
Starch isolation aims to be hydrolyzed into sugar.
The sample as a source of starch used is banana
weevil. From 1000 grams of Saba Banana Hump
obtained 157 grams of starch banana weights that are
milky white. Then a qualitative test was carried out
on the starch that had been obtained using iodine
solution. Iodine test is used to detect the presence of
starch.
The iodine test is carried out by means of a starch
sample inserted into the test board, adding one drop
of aqueous iodine solution (Widyaningsih, Kartika
and Tri Nurhayati, 2012). Then mixed evenly. Test
positive iodine for starch with the appearance of blue
after adding iodine to the sample. Starch that binds to
iodine will produce a blue color and this property can
be used to analyze the presence of starch. This is
caused by the structure of the spiral starch molecule
so that it will bind to the iodine molecule and form a
blue color (Winarno, 2004).
Figure 2: Qualitative Test of Saba Banana Hump.
3.2 Analysis of Saba Banana Hump
Starch using FT-IR Spectroscopy
Functional group analysis in Saba Banana Hump
starch and commercial starch using FT-IR
spectrocopy showed almost the same wave number
and spectrum shape. Spectrum results and wave
numbers can be seen in Figure 3.
Observed at a glance, the FT-IR spectra pattern of
saba banana hump starch and commercial starch
above shows the number and location of peaks that
are relatively the same. Thus it can be interpreted that
the results of insulation are starches.
From FT-IR spectra of saba banana hump starch
and commercial starch, each absorption peak at wave
numbers 3336 cm
-1
and 3286 cm
-1
is associated as OH
strain vibrations of alcohol in starch molecules,
followed by absorption peaks at wave numbers of
2931 cm
-1
is associated as the CH stretch vibration of
the alkane chain. Whereas absorption at wave number
1639 cm
-1
is associated as O-H (H
2
O), then
absorption at wave numbers 1408 cm
-1
and 1350 cm
-
1
indicates the presence of C-H groups (Wijaya et al.,
2019). In addition, uptake at wave numbers of 1149
cm
-1
and 1145 cm
-1
is associated as vibrational strain
of C-O-C in the starch ring (Leon, 2016).
The Effect of Inoculum Volume on Bioethanol Production from Saba Banana Hump (Musa Paradisiacal. L) Starch by Zymomonas Mobilis
using Immobilization Technique
55
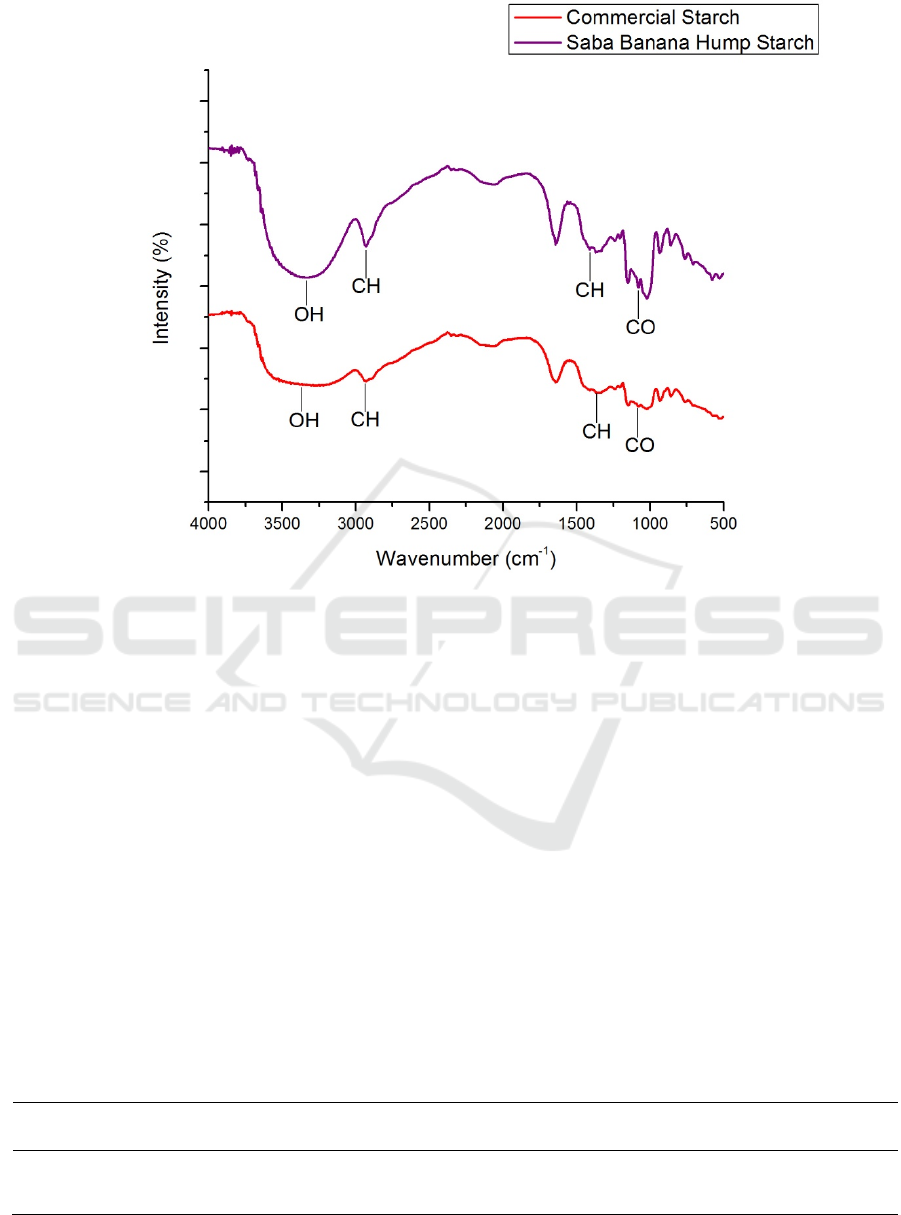
Figure 3: Results of FT-IR Spectrum of Saba Banana Hump Starch and Commercial Starch.
3.3 Starch Hydrolysis
Hydrolysis of 20 gram saba banana starch using 25%
HCl solution and 30% NaOH produced 14.01%
glucose solution. Hydrolysis of saba banana starch
using 25% HCl solution at 90°C produces glucose
solution. The purpose of hydrolysis is to break down
carbohydrates into simple sugars so that they can be
used in the fermentation process. In this research, HCl
acid is used as a catalyst in the hydrolysis process.
HCl catalysts produce higher glucose when compared
with H
2
SO
4
catalysts. This is due to H
2
SO
4
being
combustible while HCl is not so that the use of HCl
catalyst is more optimal in producing reducing
sugars. The acid produces H
+
ions and binds with H
2
O
to form H
3
O
+
will break the gilosidic bonds in
amylose and amylopectin to form simple monomers
(Balat, Balat and Öz, 2008).
3.4 Cell Immobilization Process
The process of immobilization of zymomonas
mobilis bacteria is to add a bacterial inoculum into the
nutrient media with variations in the bacterial
inoculum 5%, 10%, and 15%. After the next
incubation process 4% alginate solution will be added
in a ratio of 1:1 to a 2% CaCl solution which results
in a compact and round solid beads. Various studies
have shown that calcium alginate protects culture cell
immobilization better by increasing bacterial
resistance than without immobilization (Anal and
Singh, 2007). Alginates can form gels (egg-box
formations), films, manic (beads), pellets,
microparticles, and nanoparticles (Natalia, 2015).
Table 1: Bioethanol results from the fermentation of Saba banana hump starch.
No Zymomonas mobilis Inoculum (%) Bioethanol Content (%) Productivity (g/L.Hours)
1 5 68.93 2.49
2 10 79.01 2.60
3 15 77 2.66
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
56
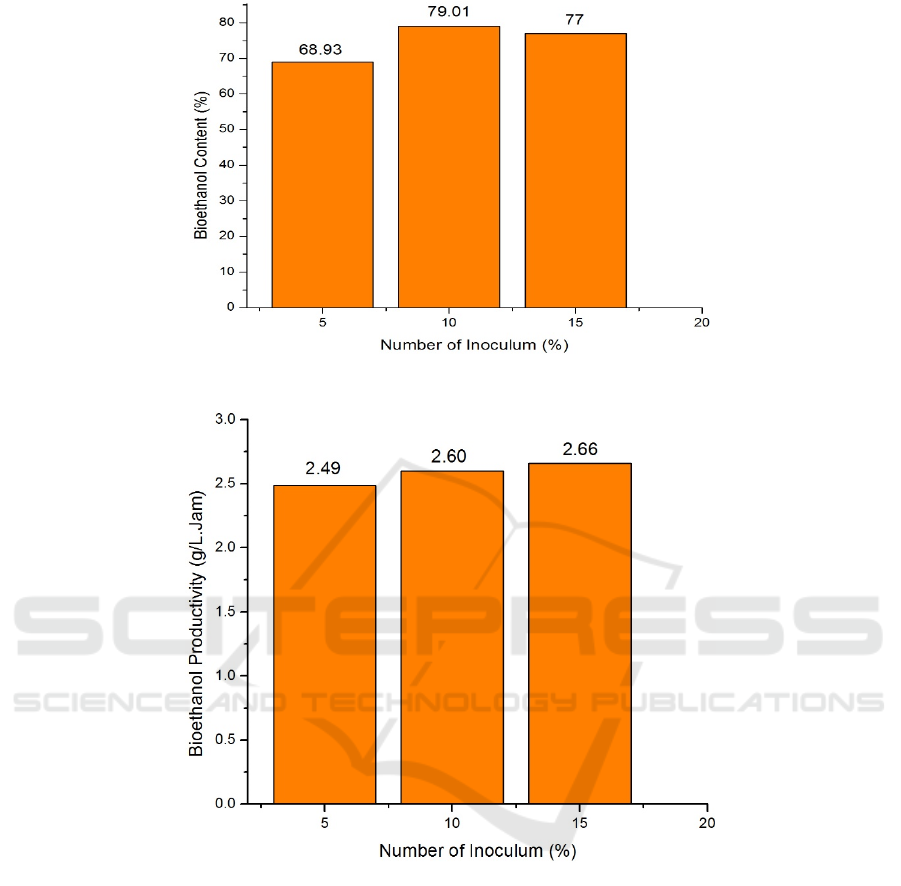
Figure 4: Graph of Bioethanol Levels.
Figure 5: Graph of Bioethanol Productivity.
3.5 Fermented Banana Starch Results
Kepok Banana
The fermentation process is carried out using an
immobilized Zymomonas Mobilis inoculum. Where
with the treatment of variation inoculum Zymomonas
Mobilis as much as 5%, 10%, and 15%. Furthermore,
the separation of bioethanol from the fermentation
solution using a rotary evaporator. Then the
fermentation results are tested using Gas
Chromatography. In the variation of zymomonas
mobilis inoculum as much as 5%, 10%, and 15%, the
results were fluactative yield. The results of
bioethanol can be seen in table 1.
From Figure 4 the diagram of the effect of
inoculum volume on bioethanol levels can be
explained that at 5% inocolum volume of bioethanol
produced is 68.93%, whereas an increase in
bioethanol levels in 10% inoculum volume with a
duration of 79.01%. However, there was a decrease in
levels of bioethanol at 15% inoculum volume
treatment by 77%. Hisreidi, (2016) said the higher
inoculum volume indicates that more and more
populations of Zymomonas mobilis bacteria are
fermenting and as a result the higher levels of
bioethanol produced (Hisreidi, 2016).
Figure 5 is a diagram of bioethanol productivity
with Zymomonas Mobilis inoculum variations of 5%,
The Effect of Inoculum Volume on Bioethanol Production from Saba Banana Hump (Musa Paradisiacal. L) Starch by Zymomonas Mobilis
using Immobilization Technique
57

10%, and 15%. Bioethanol productivity in
zymomonas mobilis inoculum variation of 5%, 10%,
and 15% showed increased results, namely 2.49%,
2.6%, and 2.66%. The highest results were obtained
in the zymomonas mobilis inoculum variation 15%
by 2.66%, while the lowest results were obtained in
the zymomonas mobilis inoculum variation 5% by
2.49%. It can be concluded that the higher the level
of bioethanol, the higher the value of bioethanol
productivity
4 CONCLUSIONS
Saba banana hump can produce starch that has a white
color. Starch from kepok banana weevil hydrolyzed
using acid to produce glucose as much as 14.01%
with 20 grams of starch content. The highest
bioethanol content is 79.01% and the highest
productivity that obtain was 2.66 g/L.Hours.
REFERENCES
Anal, A. K. and Singh, H. (2007) ‘Recent advances in
microencapsulation of probiotics for industrial
applications and targeted delivery’, Trends in Food
Science and Technology, pp. 240–251. doi:
10.1016/j.tifs.2007.01.004.
Atwell, W.A., Hood, L., Lineback, D., Varriano-Morston,
E., Zobel, H. (1988) ‘The terminology and
methodology associated with basic starch phenomena’,
Cereal Food World, 33, pp. 306–311.
Balat, M., Balat, H. and Öz, C. (2008) ‘Progress in
bioethanol processing’, Progress in Energy and
Combustion Science, pp. 551–573. doi:
10.1016/j.pecs.2007.11.001.
Geeta, M. S. G. & G. S. (2007) ‘Effectivenes of Fungal
Pretreament of Agro Residues on Etanol Production by
Yeast and Zymomonas mobilis’, 2, pp. 301–304.
Gunasekaran, P. and Chandra Raj, K. (1999) ‘Ethanol
fermentation technology - Zymomonas mobilis’,
Current Science.
Hisreidi (2016) Pengaruh Volume Inokulum Pada Produksi
Bioetanol Dari Kulit Pisang Kepok Kuning (Musa
paradisiaca L. var. Kepok Kuning) Menggunakan
Zymomonas mobilis Dengan Metode Solid State
Fermentation (SSF). Universitas Sanata Dharma.
Kusumaningati, M. A., Nurhatika, S. and Muhibuddin, A.
(2013) ‘Potensi Kapang Aspergillus sp. dalam Proses
Hidrolisis untuk Produksi Etanol dari Sampah Sayur
dan Buah Pasar Wonokromo Surabaya’, Makalah Orasi
Ilmiah.
Leon (2016) AC SC, Food Hydrocolloids. Elsevier Ltd. doi:
10.1016/j.foodhyd.2016.01.008.
Lin, Y. and Tanaka, S. (2006) ‘Ethanol fermentation from
biomass resources: Current state and prospects’,
Applied Microbiology and Biotechnology, pp. 627–642.
doi: 10.1007/s00253-005-0229-x.
Natalia, S. . (2015) Viabilitas enkapsulasi sinbiotik isolat
BAL dengan berbagai bahan enkapsulasi selama masa
simpan dan simulasi asam lambung. Universitas
Sumatera Utara.
Pacifico, C., Wu, W. and Fraley, M. (2001) ‘Sensitive
substance encapsulation’, US Patent 6,251,478, 1(12),
pp. 1–8.
Rodrigues, A. and Emeje, M. (2012) ‘Recent applications
of starch derivatives in nanodrug delivery’,
Carbohydrate Polymers, pp. 987–994. doi:
10.1016/j.carbpol.2011.09.044.
Setiadji, S. et al. (2017) ‘Alternatif Pembuatan Biodiesel
Melalui Transesterifikasi Minyak Castor (Ricinus
communis) Menggunakan Katalis Campuran Cangkang
Telur Ayam dan Kaolin’, Jurnal Kimia VALENSI, 3(1),
pp. 1–10. doi: 10.15408/jkv.v3i1.4778.
Widyaningsih, S., Kartika, D. and Tri Nurhayati, Y. (2012)
‘PENGARUH PENAMBAHAN SORBITOL DAN
KALSIUM KARBONAT TERHADAP
KARAKTERISTIK DAN SIFAT BIODEGRADASI
FILM DARI PATI KULIT PISANG’, Molekul, 7(1), p.
69. doi: 10.20884/1.jm.2012.7.1.108.
Wijaya, C. et al. (2019) ‘Heliyon Isolation and
characterization of starch from Limnophila aromatica’,
Heliyon. Elsevier Ltd, 5(October 2018), p. e01622. doi:
10.1016/j.heliyon.2019.e01622.
Winarno, F. G. (2004) Kimia Pangan dan Gizi
. Jakarta: PT.
Gramedia.
Wu, W., Roe, W. and Gimino, V. (2000) ‘Low melt
encapsulation with high laurate canola oil’, US Patent
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
58
