
Bioethanol Production from Lindur Fruit (Burguiera Gymnorrhiza)
Strach with Variation of Inoculum Volume of
Zymomonas Mobilis
Hamdan Azhari
1
, Emma Zaidar Nasution
2*
and Rumondang Bulan Nasution
2
1
Postgraduate Chemistry Study Programme, Universitas Sumatera Utara, Jl. Bioteknologi No. 1, Medan, Indonesia
2
Department of Chemistry, Universitas Sumatera Utara, Jl. Bioteknologi No. 1, Medan, Indonesia
Keywords: Acid Hydrolysis, Bioethanol, Lindur Fruit, Strach, Zymomonas mobilis.
Abstract: Bioethanol is the fermentation product of hydrolyzed carbohydrates by using acids or enzymes. Commonly,
bioethanol uses fermented microbes, one of them is bacterium Zymomonas mobilis. Lindur ( Burguiera
gymnorrhiza ) is the fruit of one kind mangrove plant which are not fully utilized. One of the chemical content
of lindur Fruit is carbohydrate 23.53 %, it can be used for the production of bioethanol which uses
fermentation of carbohydrates. Isolation of starch which is one kind of carbohydrates from lindur fruit by
precipitation the starch using water. Pati will be hydrolyzed using HCl 20 % to obtain a solution of glucose
around 7.49 %. after that, fermentation carried out using a different variation of the number of inoculums (5,
10 and 15 % (v/v)). Bioethanol obtained from the fermentation process will be measured using Gas
Chromatography (GC ), density, acidity, and evaporation residue also tested. The result shows the highest
content of bioethanol is 43.75 %.
1 INTRODUCTION
Lindur fruit or Burguiera gymnorrhiza is the fruit of
one kind mangrove plant. This mangrove plant grows
a lot in tropical regions, especially Indonesia. These
plants grow in the coastal area, it aims to prevent
surface erosion by sea waves (abrasion)
Lindur fruit which is not fully utilized by many
people. This fruit has a carbohydrate content of
around 23.53 % (De, 2005). Carbohydrates are
natural polymers that are abundant in nature, one type
of carbohydrate is starch. Starch is a glucose
homopolymer with α-glycosidic bonds. Lindur starch
has an amylose content of about 31.56 % and an
amylopectin content of about 26.17 % (Jacoeb et al.,
2014). Starches consisting of glucose can be used for
bioethanol production.
Bioethanol can be produced using microbial help.
Zymomonas mobilis is a bacterium that can ferment
glucose and fructose (Gunasekaran and Chandra Raj,
1999) (Geeta, 2007). Bioethanol productivity
obtained from zymomoas mobilis is higher when
using the Entner-Duodoroff pathway (Obire, 2005)
(Tripetchkul S.Z.D Hilary, 1998). Zymomonas
mobilis is not harmful to humans and is often used as
a natural inoculum to make traditional alcoholic
drinks. Most of Zymomonas strains (90 %) can grow
at pH 3.5. But it does not grow at pH 3.05 or lower.
Because Zymomonas is rather thermolabile, the best
condition for Zymomonas mobilis growth is at
temperatures between 25
o
C and 30
o
C; 74 % of
strains grow at 38
o
C, but the growth will rarely occur
at 40
o
C.
Zymomonas mobilis has a tolerance to high
substrate and product concentrations. Some types of
Zymomonas can tolerate up to 30-40 % glucose and
13% (weight/volume) ethanol. This bacteria has a
high tolerance to ethanol among the other bacteria,
only the majority of bacterial growth is inhibited by
ethanol concentrations of 1-2 % (weight/volume). As
an explanation, the main protective function comes
from hopanoids, which are pentacyclic triterpenoids,
which are widely present in the Zymomonas mobilis
cell membrane. Most likely, amphiphilic substances,
such as sterols, stabilize Zymomonas mobilis cell
membranes against dissolving with ethanol (Yanase,
2014).
This research aimed to determine the content of
bioethanol produced from zymomonas mobilis
fermentation in starch from acid hydrolyzed.
Azhari, H., Zaidar Nasution, E. and Bulan Nasution, R.
Bioethanol Production from Lindur Fruit (Burguiera Gymnorrhiza) Strach with Variation of Inoculum Volume of Zymomonas Mobilis.
DOI: 10.5220/0010133100002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 47-52
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
47

2 METHODOLOGY
2.1 Preparation Lindur Fruit
The fruit obtained is then peeled and cleaned with
clear water, then cut into cubes and then put into a
bucket and soaked with 0.2 % Na
2
S
2
O
5
for 12 hours.
Then blend until smooth by adding aquadest 1:5
(weight/volume) then let stand for 24 hours.
Furthermore, starch is separated from the solution and
roasted at a temperature of 45
o
C for 24 hours.
2.2 Fourier Transforms Infrared
Spectroscopy
The starch was prepared into pulp. Pulp slurry was
examined in a thin film placed between flat plates of
salt. The test was carried out by pinning the resultant
film on the sample container. Then the film was
placed on a plate in the direction of infrared light. The
result will be recorded periodic paper in the form of a
flow curve of 4000-200 cm
-1
waves with intensity.
2.3 Starch Hydrolysis
10 g of starch obtained was then put into a 250 ml
glass beaker, and then added with 100 ml 25% HCl
and covered with alumanium foil. Then it was heated
at 80
o
C while stirring for 30 minutes. After that, it
was cools down, the hydrolyzate was adjusted to
neutral pH using 30 % NaOH.
2.4 Preparation YEPD Media (Yeast
Extract Pepton Dextrose)
The preparation of YEPD media is by dissolving 4 g
Yeast extract, 2 g KH
2
PO
4
(s)
, 3 g (NH
4
)
2
SO
4
(s)
, 1 g
MgSO
4
.7H
2
O
(s)
, 3.6 g Pepton and 2% Bacto agar
with 1000 ml Aquadest. Then heated on a hotplate
until it was clear yellow.
2.5 Zymomonas Mobilis Bacteria
Culture
Z. Mobilis bacterial culture is carried out in a sterile
place near or around a burning Bunsen fire so that
there are no contaminants that inhibit the growth of
Z. Mobilis bacteria. Culture was carried out by
inserting YEPD media into a petri dish, then 1 ose
was taken from isolate Z. mobilis and then etched on
a petri dish containing YEPD media. When the petri
dish was closed and wrapped in plastic wrap, it was
then incubated at 30
o
C for 24 hours.
2.6 Hydrolyzed Fermentation using
Inoculum Z. Mobilis
Fermentation using inoculum Z. Mobilis was done by
dissolving 4 g Yeast extract, 2 g KH
2
PO
4
(s)
, 3 g
(NH
4
)
2
SO
4
(s)
, 1 g MgSO
4
.7H
2
O
(s)
, 3.6 g Pepton and
2% agar Bacto with 1000 ml of hydrolyzate. Then
sterilized using an autoclave for 2 hours at 121
o
C.
After the hydrolyzate was cooled, 5 %, 10 % and 15
% inoculum Z. Mobilis was added. Then it was tightly
closed using alumanium foil and plastic wrap and put
in a shaker incubator for 21 hours at 30
o
C with 100
rpm speed.
2.7 Separation of Bioethanol from
Fermentation Solutions
500 ml fermentation solution was put into a 1000 ml
rotary evaporator flask then CaO was added to the
fermentation solution at a ratio of 1: 2 (g / ml) and
then in the rotary evaporator at 78
o
C for 1 hour. Then
the distillate obtained was test for ethanol qualitative.
Figure 1: Lindur Fruit.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
48
2.8 Bioethanol Density Test
Bioethanol density test was carried out using a
pycnometer, where the dry, clean and empty
pycnometers weighed, then filled with water/
aquadest, and then it was dried and cleaned.
Furthermore, weighed with an analytical balance to a
constant weight. The same step was done by using a
distillate (bioethanol).
Density (g∕ml)=(a-w)/(b-w)
a = weight of empty pycnometer + sample
b = weight of empty picnometer + water
w = weight of the empty pycnometer
2.9 Determination of Bioethanol
Content
Determination of obtained bioethanol content by
using bioethanol density conversion tables with
bioethanol content.
3 RESULTS AND DISCUSSIONS
3.1 Starch Isolation Results from
Lindur Fruit
Starch isolation from lindur fruit was carried out by
precipitating starch in water overnight, so that starch
from lindur fruit has a brownish white color. Starch is
a type of carbohydrate which is a glucose
homopolymer with α-glycosidic bonds and there are
many in all plants, one of which is fruit. Starch is in
the cortical tissue in the fruit which is located in the
xylem surrounded by phloem (Seknun, 2012) so that
by destroying the fruit will damage the cortical tissue
so that the starch of the fruit can be removed. Where
the physical properties of starch that can not dissolve
in aquadest it will precipitate starch at the bottom of
the solution because the molecular mass of starch is
heavier than the water molecules in the fruit juice.
3.2 Characterization using Fourier
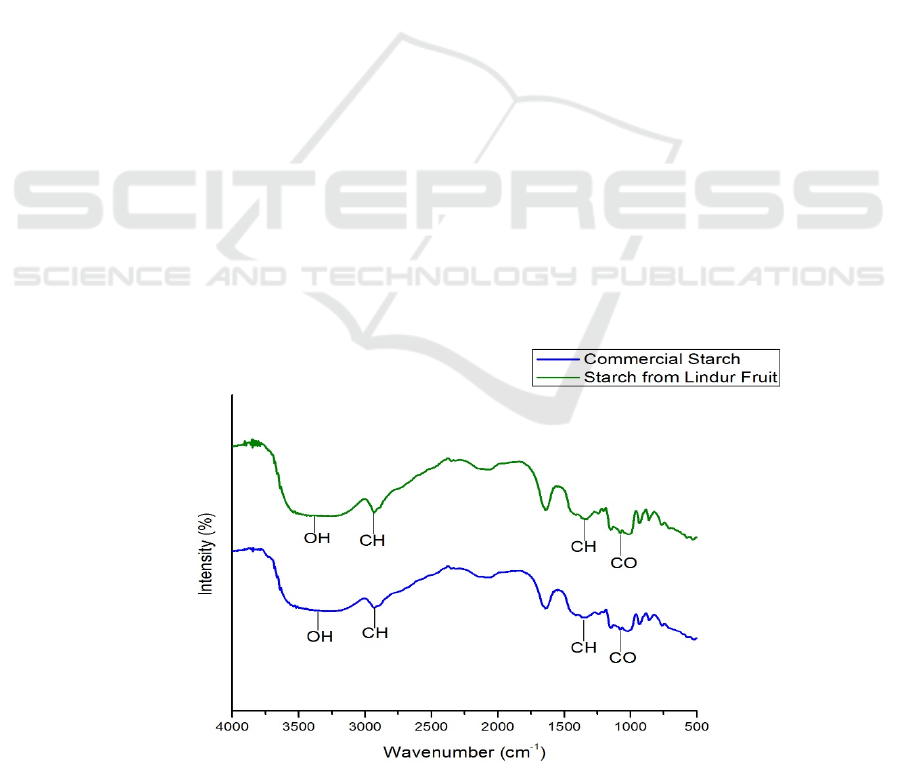
Transforms Infrared Spectroscopy
In the functional group analysis using FTIR for both
the spectrum of the fruit starch and commercial starch
showed that there was no significant difference
between the starch band of the fruit and the
commercial starch. It happened because both are
starches. From FTIR spectra, there are widening
bands in the absorption regions 3398 and 3286 cm
-1
which show the existence of OH stretch vibrations
from alcohol in the starch molecule, followed by the
CH stretch vibrations of the alkane chains in the
absorption area 2931 cm
-1
.(Estrada-León et al., 2016)
In addition, the vibrational peak was also seen in the
absorption area of 1145 cm
-1
which showed the
presence of C-O-C strain in the starch ring (Estrada-
León et al., 2016). Whereas the absorption area of
1338 and 1350 cm
-1
indicates the presence of C-H
groups (Wijaya et al., 2019).
Figure 2: Spectrum FT-IR of Commercial Starch and Starch from Lindur Fruit.
Bioethanol Production from Lindur Fruit (Burguiera Gymnorrhiza) Strach with Variation of Inoculum Volume of Zymomonas Mobilis
49
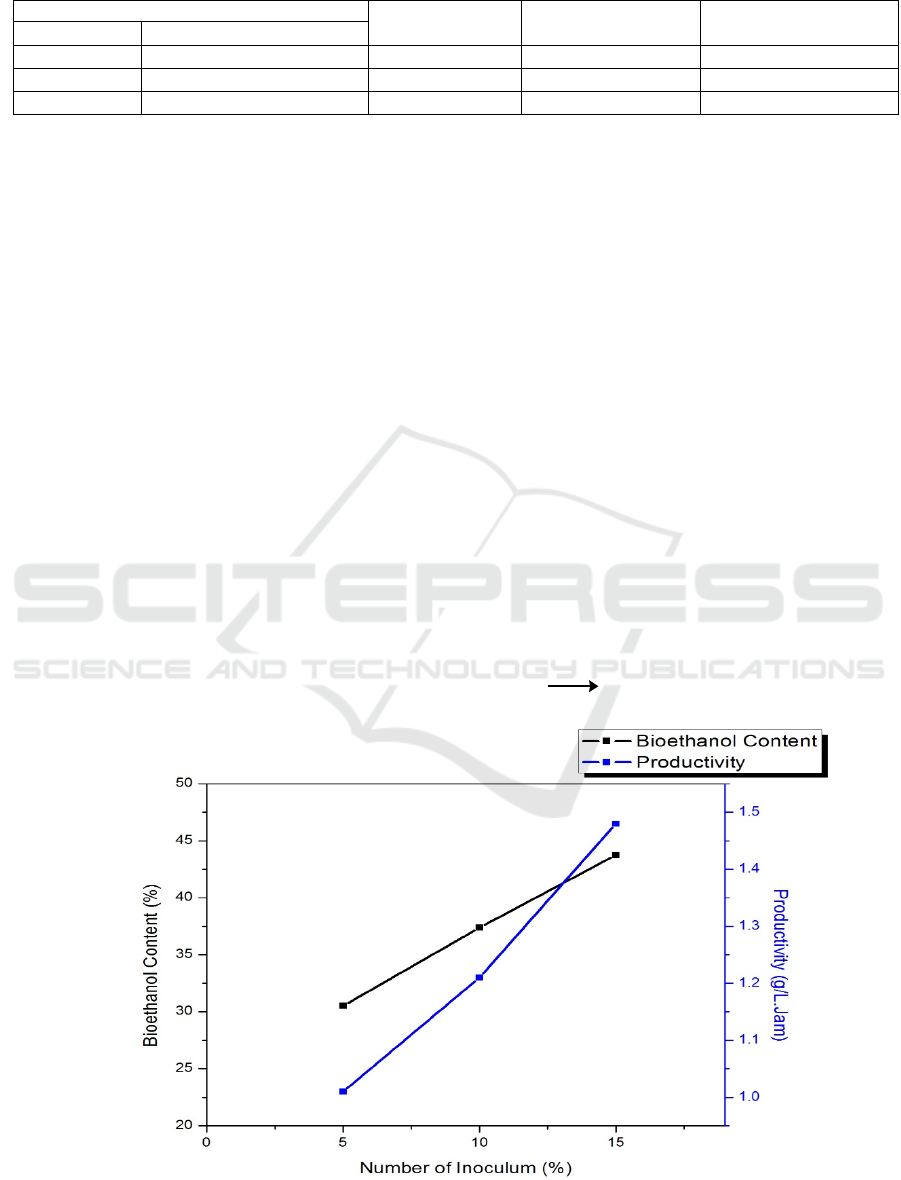
Table 1: Results of Starch Fermentation from Hydrolyzed Lindur Fruit.
Treatment
Yield (%)
Bioethanol
content (%)
Productivity (g / L.
Hours)
Starch (%) Inoculum Z. Mobilis (%)
10 5 8.8 30.53 1.01
10 10 8.6 37.38 1.21
10 15 9.0 43.75 1.48
3.3 Hydrolysis Starch by Acid
Hydrolysis of lindur fruit starch using 25 % HCl
solution and neutralization using 30 % NaOH
solution to obtain a glucose solution of about 7.5 %.
The hydrolysis of starch from the fruit yields 7.5 %
glucose from 10% starch content. The added acid can
hydrolyze because it can form hydroxonium ions
(H
3
O
+
) which are electrophilic so that they attack the
oxygen atom in the glycosidic α-1,4 bond and
hydrolyze the glucosidic bond. Then the electrons in
one of the carbon-oxygen bonds move to the oxygen
atom and produce an unstable high-energy
carbocation intermediary. Furthermore, intermediate
carbocation reacts with water, which leads to the
regeneration of hydroxyl groups (Hoover, 2000).
3.4 Fermented Hydrolyzed Starch
Results of fermentation of hydrolysis solution of
starch fruit will be distilled using a rotarievaporator
to separate the bioethanol obtained by boiling point
differences. The bioethanol content obtained will be
determined using gas chromatography (GC).
Zymomonas mobilis is widely used as a
fermentation bacterium, which converts glucose,
sucrose, and fructose into ethanol. Like Z. mobilis,
Saccharomyces cerevisiae naturally consumes hexose
sugar (for example, glucose, fructose).
The metabolism in Zymomonas is different from
the metabolism of Saccharomyces in which glucose
becomes pyruvate through the Enbden-Meyerhof-
Parnas (EMP) pathway; ethanol is then formed from
pyruvate. Instead, Zymomonas ferments sugar
through the ED pathway, forming pyruvate from
gluconate. As in Saccharomyces, the released
pyruvate is decarboxylation, producing acetaldehyde
and CO
2
, after which acetaldehyde is reduced to
produce ethanol.
When the formation of ATP through the EMP and
ED pathways was compared, it was found that EMP
produced 2 moles of ATP per mole of glucose,
whereas the ED pathway produced 1 mole of ATP per
mole of glucose. Thus, ATP cells result in less
glucose in Zymomonas metabolism than in yeast. The
equation describing molar fermentation is as follows:
1mol C
6
H
12
O
6
1,93 mol C
2
H
5
OH + 1,8 mol CO
2
+ 0,053 mol CH
3
CHOHCOOH
Figure 3: Fermentation Results Chart.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
50
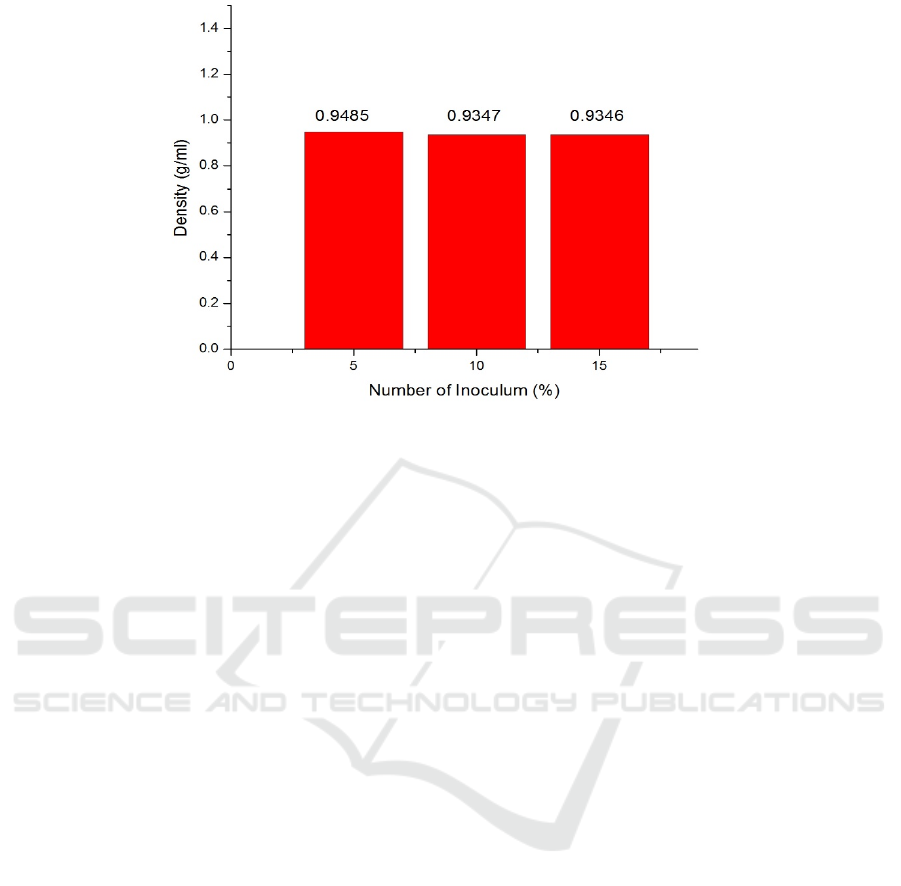
Figure 4: Bioethanol Density Graph.
In table 1 above, it can be seen that the % yield
obtained from the distillation of fermentation
solutions using 10 % starch content ranges from ± 9
% with bioethanol content ranging from 30-40 %.
Whereas in Figure 3 can be seen in the variation
of the number of Zymomonas mobilis bacteria
inoculums there is an increase in the productivity of
bioethanol where in the starch variation of 10 % the
highest productivity is 1.48 g / L. Hours. This is in
accordance with (Fajrin, Amraini and Muria, 2008) ,
which with an increase in the number of inoculums
will increase the productivity or bioethanol content
produced, according to (Kusumaningati, Nurhatika
and Muhibuddin, 2013) with an increase in the
number of inoculums, it will increase its bioethanol
levels due to more microorganisms that can utilize
reducing sugars. According to (Prescott, 1981) there
are two factors that influence the occurrence of
increased bioethanol content, namely the number of
substrates (sugar) and the number of microbes.
3.5 Density Test
In Fig. 4 above it can be seen that the highest density
value on the variation of starch is 10 % and the
amount of inoculum is 5 % with a density of 0.9485
g / ml while the lowest density with an amount of
inoculum of 15 % is 0.9346 g / ml. This is due to the
imperfect distillation process so that bioethanol is still
mixed with water where the pure bioethanol content
has a density of 0.798 g / ml but the density value
obtained exceeds the density of pure bioethanol. So it
can be concluded that the density value has decreased
with the addition of the number of inoculum.
This decrease in density is due to the amount of
inoculum which can increase bioethanol levels or
productivity. High bioethanol content will have a low
density value. So it can be explained that the density
value of bioethanol is inversely proportional to the
bioethanol contentwhere the higher the bioethanol
content, the lower the density value.
4 CONCLUSIONS
Lindur fruit can produce starch that has a brownish
white color. Lindur fruit starch hydrolyzed using acid
produces a glucose solution of about 7.5 % with 10 %
starch content. Fermentation of hydrolysis solution of
lindur fruit starch showed productivity of 1.48 g /
L.Hours
REFERENCES
De, F. J. (2005) Ditemukan Buah Bakau Sebagai Makanan
Pokok. Available at: http://www.ebook.pangan.com.
Estrada-León, R. J. et al. (2016) The effect of isolation
method on properties of parota (Enterolobium
cyclocarpum) starch, Food Hydrocolloids. Elsevier
Ltd. doi: 10.1016/j.foodhyd.2016.01.008.
Fajrin, I., Amraini, S. Z. and Muria, S. R. (2008) ‘Pengaruh
Volume Inokulum pada Produksi Bioetanol dari
Limbah Kulit Nanas Menggunakan Zymomonas
Mobilis dengan Metode Solid State Fermentation ( SSF
) Ikhsan Fajrin , Said Zul Amraini *, Sri Rezeki Muria
Laboratorium Rekayasa Bioproses Jurusan Teknik
Kimia’, I, pp. 1–5.
Bioethanol Production from Lindur Fruit (Burguiera Gymnorrhiza) Strach with Variation of Inoculum Volume of Zymomonas Mobilis
51

Geeta, M. S. G. & G. S. (2007) ‘Effectivenes of Fungal
Pretreament of Agro Residues on Etanol Production by
Yeast and Zymomonas mobilis’, 2, pp. 301–304.
Gunasekaran, P. and Chandra Raj, K. (1999) ‘Ethanol
fermentation technology - Zymomonas mobilis’,
Current Science.
Hoover, R. (2000) ‘Acid-treated starches’, Food Reviews
International. doi: 10.1081/FRI-100100292.
Jacoeb, A. M. et al. (2014) ‘Pembuatan Edible Film Dari
Pati Buah Lindur Dengan Penambahan Gliserol Dan
Karaginan Edible Film from Lindur Fruit Starch with
Addition of Glycerol and Carrageenan’, Jphpi.
Kusumaningati, M. A., Nurhatika, S. and Muhibuddin, A.
(2013) ‘Potensi Kapang Aspergillus sp. dalam Proses
Hidrolisis untuk Produksi Etanol dari Sampah Sayur
dan Buah Pasar Wonokromo Surabaya’, Makalah Orasi
Ilmiah.
Obire, O. (2005) ‘Activity of Zymomonas species in palm-
sap obtained from three areas in Edo State, Nigeria.’, J.
Appl. Sci. Environ. Mgt.
Prescott, S. . (1981) Industrial Microbiology. New York:
Mc. Grow-Hill Book Co Ltd.
Seknun, N. (2012) ‘Pemanfaatan Tepung Buah Lindur (
Bruguiera Gymnorrhiza ) Dalam Pembuatan Dodol
Sebagai Upaya Peningkatan Nilai Tambah Oleh :
Niswani Seknun Departemen Teknologi Hasil
Perairan’, pp. 1–71.
Tripetchkul S.Z.D Hilary (1998) ‘strategies for improving
ethanol production using zymomonas mobilis’, Devel.
in Agricultural & Biological Chem, 2, pp. 41–55.
Wijaya, C. et al. (2019) ‘Isolation and characterization of
starch from Limnophila aromatica’, Heliyon. Elsevier
Ltd, 5(5), p. e01622. doi:
10.1016/j.heliyon.2019.e01622.
Yanase, H. (2014) ‘Zymomonas’, Encyclopedia of Food
Microbiology: Second Edition, 3, pp. 856–863. doi:
10.1016/B978-0-12-384730-0.00365-7.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
52
