
Synthesis of Antimicrobial and Emulsifier Compunds through
Hydroxyl Group Esterification of Oxidized Ricinoleic Acid
Rifqah Azzahra Naulidia, Sri Handayani, Siswati Setiasih and Sumi Hudiyono
Department of Chemistry, Universitas Indonesia, Depok, Indonesia
Keywords: Ricinoleic Acid, Oxidation, Esterification, Emulsifier, Antimicrobial Agents
Abstract: In this study, ester synthesis from commercial oxidized ricinoleic acid using various carboxylic acid was
conducted. The double bond in ricinoleic acid was oxidized using KMnO
4
in alkaline condition to form two
hydroxyl groups. Oxidized ricinoleic acid was then esterified chemically using palmitic acid, decanoic acid,
and butyric acid by ZnCl
2
as catalyst with the molar ratio of oxidized ricinoleic acid to carboxylic acid was
3:1. Esters produced were characterized using FTIR examined as emulsifier and the antimicrobial activity.
The results showed that each ester product gave absorption band C=O ester at the range of 1600 cm
-1
-1720
cm
-1
. The highest conversion percentage of esterification was obtained by oxidized ricinoleic acid-palmitic
acid esters with the value of 75%. Simple emulsifier test was performed for each ester and the result showed
that esters were able to maintain an emulsion form approximately 24 hours with water-in-oil emulsion (w/o)
type. The antimicrobial activity test of esters gave positive results in the presence of inhibition zone to the
growth of Propionibacterium acnes and Staphylococcus epidermidis. The highest antimicrobial activity
against P. acnes and S. epidermidis was produced by oxidized ricinoleic acid-decanoic acid esters.
1 INTRODUCTION
Ricinus communis L. is one of industrial plant that
has bright prospects to be developed in
Indonesia. Ricinus communis L. seeds contain 46%
oil, which are known as castor oil. Castor oil content
is dominated by ricinoleic acid (89,5% of total fatty
acids). Ricinoleic acid (C18) is an unsaturated fatty
acid with one double bond at carbon-9 (C
9
) and
hydroxyl group in the side chain (R) position of
carbon-12 (C
12
). Other than double bonds and
hydroxyl groups, ricinoleic acid has carboxylic
groups which make this fatty acid can be reacted
through various reactions to get its derivative
product. One form of its derivative product is
oxidized ricinoleic acid esters (Kajikawa et al.,
2016).
The double bond in ricinoleic acid can be
oxidized by using a strong oxidizer to produce diol.
The addition of diol groups can increase polarity of
ricinoleic acid which makes this compound to
reduce surface tension, because it can combine polar
and nonpolar phase. The hydroxyl groups of
oxidized ricinoleic acid can be esterified by
carboxylic acids to form oxidized ricinoleic acid
esters which can be used as an emulsifier due to its
properties.
Previous study showed that derivatives of fatty
acid in the form of long chain esters could be used to
be skin barrier (Pérez et al., 2016). In this study,
oxidized commercial ricinoleic acid esters were
synthesized by esterification reaction with palmitic
acid, decanoic acid, and butyric acid using ZnCl
2
as
catalyst. Esterification products were then
characterized using FT-IR and examined its ability
as emulsifier and its antimicrobial activity against
Propionibacterium acnes and Staphylococcus
epidermidis.
2 EXPERIMENT
2.1 Materials
Materials used in this study were commercial
ricinoleic acid, sodium hydroxide, potassium
permanganate, chloroform, Wijs solution, potassium
iodide, sodium thiosulphate, starch, palmitic acid,
decanoic acid, butyric acid, zinc chloride, n-hexane,
eosin, phenolphthalein, methanol, antibiotic
clindamycin, nutrient broth, nutrient agar,
42
Azzahra Naulidia, R., Handayani, S., Setiasih, S. and Hudiyono, S.
Synthesis of Antimicrobial and Emulsifier Compunds through Hydroxyl Group Esterification of Oxidized Ricinoleic Acid.
DOI: 10.5220/0010133000002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 42-46
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

Propionibacterium acnes and Staphylococcus
epidermidis from Universitas Indonesia.
2.2 Methods
2.2.1 Oxidation of Commercial Ricinoleic
Acid
Oxidation was carried out by mixing commercial
ricinoleic acid, NaOH 2 M, and KMnO
4
0.5 M. This
mixture was stirred using magnetic stirrer at
temperature 25
0
C-27
0
C for 30 minutes. After 30
minutes, there would be two phases. Organic phase
was then separated and filtered.
2.2.2 Determination of Iodine Value
Oxidized ricinoleic acid was mixed with chloroform
and Wijs solution, then left for 30 minutes in dark
place. After 30 minutes, KI 15% and distilled water
were added. The mixture was then titrated using
sodium thiosulphate 0.1 N until the colour of
mixture turn to yellowish. Starch was added and the
mixture was titrated again by sodium thiosulfate
until the colour turned clear (Goud et al., 2006).
2.2.3 Esterification
Esterification was carried out by reacting oxidized
ricinoleic acid in n-hexane as solvent with palmitic
acid on reflux system at temperature 60
o
C for 6
hours using ZnCl
2
as catalyst. ZnCl
2
used in this
reaction used in this reaction was 0.3 % of the total
mass substrate (w/w substrate). The mol ratio of
oxidized ricinoleic acid to palmitic acid used in this
reaction was 3:1 (mol/mol) (Gonçalves et al., 2011).
The same steps were performed using decanoic acid
and butyric acid. Ester products were then purified
by extraction using n-hexane and methanol.
2.2.4 Determination of Conversion
Percentage
The conversion percentage was determined by
titrating the organic phase that has been obtained
from extraction in n-hexane using 0.1 N NaOH and
phenolphthalein as indicator (Handayani et al.,
2012).
2.2.5 FT-IR Analysis
Commercial ricinoleic acid, oxidized commercial
ricinoleic acid, and all esters were characterized
using FTIR.
2.2.6 Simple Emulsifier Test and
Determination of Emulsion Type
Emulsifier test was performed by mixing 0.1 g
esters, oil, and water according to Table 1. The
mixtures were shaken, and the emulsion stability
was observed up to 24 hours. The emulsion type
determination was observed under microscope using
eosin as indicator.
Table 1: Variation of Oil and Water Composition in the
Making of Emulsion.
Oil in Wate
r
Water in Oil
Tube
Numbe
r
1 2 3 4 5 Tube
Numbe
r
1 2 3 4 5
Water
(ml)
2 2 2 2 2 Water
(drops)
2 4 6 8 10
Oil
(drops)
2 4 6 8 10 Oil (ml) 2 2 2 2 2
2.2.7 Antimicrobial Activity Assay
Disc diffusion method was performed to determine
antimicrobial activity. An aliquot of 200 µL bacteria
suspension with density 1x10
8
cells/mL was
aseptically mixed by 20 mL nutrient agar in a sterile
petri dish. The sterile paper disc (± 6 mm in
diameter) was placed on top of the medium and
dropped by 4 μL sample to be tested. The medium
was then incubated at 37°C for 24 hours. The clear
zone around the paper discs were measured. The
bacteria that used in this research were
Staphylococcus epidermidis and Propionibacterium
acnes.
3 RESULT AND DISCUSSIONS
3.1 Oxidation of Commercial
Ricinoleic Acid
The double bond on commercial ricinoleic acid were
oxidized through dihydroxylation reaction, which
would produce two hydroxyl groups. In this study,
oxidation was performed in an alkaline condition. At
cold temperatures with low concentrations of
oxidizing agents, alkenes tend to form glycols. At
the end of reaction, MnO
2
was produced. The MnO
2
could be removed by filtering oxidation product.
3.2 Determination of Iodine Value
Oxidation product was identified through
determination of iodine value to testify that
oxidation has successfully carried out. The iodine
Synthesis of Antimicrobial and Emulsifier Compunds through Hydroxyl Group Esterification of Oxidized Ricinoleic Acid
43
value of oxidized ricinoleic acid before oxidation
was 45.30 mg/g and after oxidation it decreased to
17.29 mg/g. The decreased in iodine number proved
that ricinoleic acid was successfully oxidized.
3.3 Esterification
In this study, esterification reaction was carried out
on the OH group of oxidized ricinoleic acid with
palmitic acid, decanoic acid, and butyric acid using
ZnCl
2
catalyst. The ester products expected to be
produced were monoesters. The mole of oxidized
ricinoleic acid is higher than fatty acid. This referred
to Le Chatelier’s principle. The increased of
reactants amount will produce more products.
3.4 Determination of Conversion
Percentage
Esters that formed were purified by extraction using
n-hexane and methanol. The n-hexane layer was at
the top. This layer consisted of non-oxidized and
non-esterified compounds, while methanol layer is at
the bottom consisted of esters. The conversion
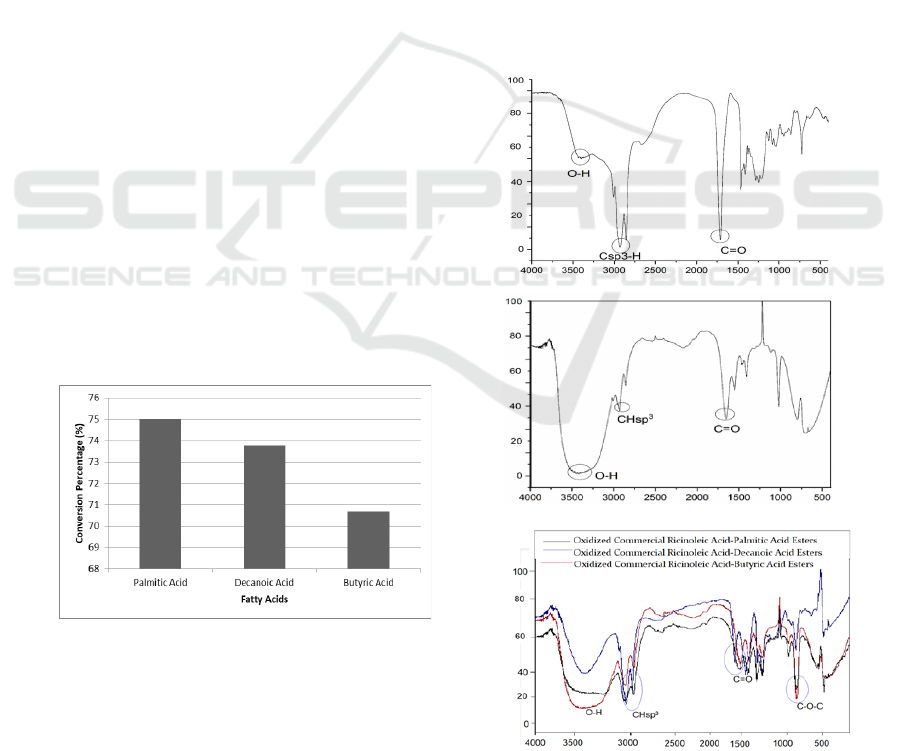
percentage is shown in Figure 1. The highest
conversion percentage was obtained by oxidized
ricinoleic acid-palmitic acid esters with the value of
75%. At the beginning of reaction, conversion
percentage of ester products will decrease along with
the chain lengthening of fatty acid used. This is
because ester products from short-chain fatty acid
are more soluble in organic solvents, such as n-
hexane. However, when the reaction is completed,
conversion percentage will remain the same
(Macierzanka & Szela̧ g, 2004).
Figure 1: The Curve of Fatty Acid Variation vs
Conversion Percentage.
3.5 FT-IR Analysis
The formed product was characterized using FTIR to
determine the success of the hydrolysis, oxidation,
and esterification reaction. IR spectrum of ricinoleic
acid, oxidation products, and ester products are
shown in Figure 2.
The FTIR spectrum of ricinoleic acid showed the
absorption peak of group C=O carboxylic acid at
wave number 1710.93 cm
-1
. In addition, there was
also the absorption peak of the -OH at wave
numbers 3408.36 cm
-1
.
Oxidized ricinoleic acid which was successfully
oxidized has the addition of two -OH groups to the
fatty acid carbon chain. The -OH group’s intensity
increased indicated the oxidation reaction has
succeeded. Oxidation products had an absorption
peak of –OH at wave number 3426.69 cm
-1
. In this
study, there was still C=C group with a small
intensity which indicated that not all double bonds
were oxidized.
IR spectra of esterification products showed
absorption peak of C-O ester group in the range of
wave numbers of 1300-1000 cm
-1
which indicated
the ester has formed. The -OH group’s intensity
decreased since esterification has occurred.
(a)
(b)
(c)
Figure 2: FTIR Spectra (a) Commercial Ricinoleic Acid,
(b) Oxidized Ricinoleic Acid, (c) Esters.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
44

3.6 Simple Emulsifier Test and
Determination of Emulsion Type
The simple emulsifier test showed that commercial
ricinoleic acid, oxidized ricinoleic acid, and esters
can act as emulsifier up to 24 hours. All products
had ability to form water in oil (w/o) type of
emulsion (Figure 3). Figure 3 showed that clear zone
surrounded red droplets. Red droplets formed were
water phase with eosin dye which dissolved because
they have same polarity, while the surrounding clear
solution was oil.
The ability of a compound as an emulsifier is
determined based on the presence of a hydrophobic
group and a hydrophilic group. The hydrophilic
group will bind to water, while the hydrophobic
group will bind to oil. If the ratio of water and oil
composition is balanced, the emulsion formed will
be stable.
Figure 3: Emulsion Types (a) Commercial Ricinoleic
Acid, (b) Oxidized Ricinoleic Acid, (c) Oxidized
ricinoleic acid-palmitic acid ester, (d) Oxidized ricinoleic
acid-decanoic acid ester, (e) Oxidized ricinoleic acid-
butyric acid ester.
3.7 Antimicrobial Activity Assay
In this study, bacteria that used were Staphylococcus
epidermidis and Propionibacterium acnes. These
two bacteria are acne causing bacteria. Table 2
shows the effectiveness classification of
antimicrobial substances (Greenwood, 1995), while
Table 3 shows antimicrobial activity of all products.
The positive control used in this study was
clindamycin 0.5%. Clindamycin is an antibiotic
commonly used for acne-causing bacteria. The
negative controls used were n-hexane and methanol
as they were used as solvents for dilution. The
purpose of using negative control was to compare
that the solvent used did not affect antimicrobial
activity (Natheer et al., 2012).
Based on Table 3, it was known that all esters
showed antimicrobial activity, while the positive
control showed no antimicrobial activity. Oxidized
ricinoleic acid-decanoic acid esters showed the most
effective antimicrobial activity against
Propionibacterium acnes and Staphylococcus
epidermidis.
Table 2: Classification of effectiveness of antimicrobial
substances (Greenwood, 1995).
Inhibit Zone
Diameter
Response of growth
barriers
> 20mm Strong
16-20 mm Medium
10-15 mm Weak
< 10 mm Not effective
Table 3: Diameter of the Inhibitory Zone of Various
Compunds against P. acnes and S. epidermidis
.
Sample Inhibitory
Zone (mm)
Effectiveness
P.
acnes
S.epide
rmidis
P. acnes S.epider
midis
Commercial
Ricinoleic
Acid
11 7 Weak Not
effective
Oxidized
Ricinoleic
Acid
20 22 Medium Strong
Oxidized
Ricinoleic
Acid-Palmitic
Acid Ester
13 12 Weak Weak
Oxidized
Ricinoleic
Acid-
Decanoic
Acid Ester
22 29 Strong Strong
Oxidized
Ricinoleic
Acid-Butyric
Acid Ester
15 20 Weak Medium
Palmitic Acid - - Not
effective
Not
effective
Decanoic
Acid
10 15 Weak Weak
Butyric Acid 27 25 Strong Strong
Clyndamycin 10 10 Weak Weak
n-hexane - - Not
effective
Not
effective
Methanol - - Not
effective
Not
effective
Antimicrobial activity is influenced by the form
of esters. Monoesters are more active as
antimicrobial agents than diesters and triesters
because the longer carbon chain the more nonpolar
Synthesis of Antimicrobial and Emulsifier Compunds through Hydroxyl Group Esterification of Oxidized Ricinoleic Acid
45

molecule. So that its solubility in water is low
(Kabara, 1984).
4 CONCLUSIONS
Synthesis of oxidized ricinoleic acid esters using the
ZnCl
2
as catalyst have been successfully performed
by showing the characteristics of the C-O ester
group at FTIR spectra. Esters could act as
emulsifiers with a type of water-in-oil emulsion and
have antimicrobial activity against
Propionibacterium acnes and Staphylococcus
epidermidis. Oxidized ricinoleic acid-decanoic acid
esters showed the highest inhibition zone against
both bacteria.
ACKNOWLEDGEMENTS
This work was funded by Hibah Publikasi
Internasional Terindeks 9 (PIT 9) Universitas
Indonesia No.
NKB.0031/UN2.R3.1/HKP.05.00/2019.
REFERENCES
Gonçalves, C. E., Laier, L. O., & Silva, M. J. d. (2011).
Novel Esterification of Glycerol Catalysed by Tin
Chloride (II): A Recyclable and Less Corrosive
Process for Production of Bio-Additives. Catal Lett,
141, 1111–1117.
https://doi.org/https://doi.org/10.1007/s10562-011-
0570-x
Goud, V. V., Pradhan, N. C., & Patwardhan, A. V. (2006).
Epoxidation of karanja (Pongamia glabra) oil by
H2O2. Journal of the American Oil Chemists’ Society,
83, 635–640.
Greenwood. (1995). Antibiotic Susceptibility (Sensitivity)
Test, Antimicrobial and Chemotherapy. McGraw-Hill
Company.
Handayani, S., Novianingsih, I., Barkah, A., & Hudiyono,
S. (2012). Enzymatic Synthesis of Sucrose Polyester
as Food Emulsifier Compound. Makara International
Colloquium of Science, 16(3), 141–148.
https://doi.org/https://doi.org/10.7454/mss.v16i3.1474
Kabara, J. J. (1984). Cosmetic and Drug Preservation.
Marcel Dekker, Inc.
Kajikawa, M., Abe, T., Ifuku, K., Furutani, K., Yan, D.,
Okuda, T., Ando, A., Kishino, S., Ogawa, J., &
Fukuzawa, H. (2016). Production of ricinoleic acid-
containing monoestolide triacylglycerides in an
oleaginous diatom, Chaetoceros gracilis. Scientific
Reports, 6(36809), 1–13.
https://doi.org/10.1038/srep36809
Macierzanka, A., & Szela̧ g, H. (2004). Esterification
Kinetics of Glycerol with Fatty Acids In The Presence
Of Zinc Carboxylates. Ind. Eng. Chem. Res., 43(24),
7744–7753.
Natheer, S. E., Sekar, C., Amutharaj, P., Rahman, M. S.
A., & Khan, K. F. (2012). Evaluation of antibacterial
activity of Morinda citrifolia, Vitex trifolia and
Chromolaena odorata. African Journal of Pharmacy
and Pharmacology, 6(11), 783–788.
Pérez, B., Bulsara, P., Rawlings, A. V., Wei, Jensen, M.
M., Wang, Z., Dickens, J., Zhang, S., Elliot, R. P.,
Glasius, M., Dong, M., Clarke, M. J., & Guo, Z.
(2016). Ultralong fatty Acy Devivatives As Ucclusive
Structure Lipids for Cosmetic Applications: Synthesis
and Characterization. ACS Sustainable Chem. Eng.,
4(12), 7137–7146.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
46
