
Synthesis of Semi Interpenetrating Polymer Network’s Hydrogel
from Bacterial Cellulose
Putri Rizky
1
, Tamrin
1
and Marpongahtun
1,2
1
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan, Indonesia
2
Laboratorium Penelitian Terpadu, Universitas Sumatera Utara, Medan, Indonesia
Keywords: Acrylic Acid, Bacterial Cellulose, Hydrogel, Interpenetrating Polymer Network, N,N’-
Methylenebisacrylamide.
Abstract: Semi interpenetrating polymer network’s (IPN) hydrogel from bacterial cellulose (BC) has been
successfully synthesized. This semi-IPN hydrogel was prepared from a suspension of BC in a solvent
system of PEG1000/NaOH in the presence of acrylic acid (AA) as monomer, potassium persulfate (KPS) as
initiator, and N,N’-methylenebisacrylamide (MBA) as crosslinker. The obtained semi-IPN hydrogel was
characterized using FT-IR and SEM, also its physical property was characterized, i.e. swelling degree and
crosslinked percentage. The swelling degree and crosslinked percentage showed the semi-IPN’s hydrogel
with the addition of 600 mg BC has the maximum value, 1338 and 46%, respectively. The FT-IR analysis
exhibited the crosslinked formation between AA and MBA with the presence of band at 1555 cm
-1
that
assigned as secondary aliphatic amine. The morphological analysis of semi-IPN’s hydrogel has a rough and
dense surface.
1 INTRODUCTION
Cellulose is an abundance natural polymer that
constructed by glucose as the monomer, and it can
be easily found as a component in the plants and
natural fibers, i.e. cotton and linen. Several bacteria
(e.g. Acetobacter xylinum) can produce cellulose
(exopolysaccharide) (Ross et al., 1991). Cellulose
that produced by microbe or bacteria is called as
bacteria cellulose which has similar chemical
properties with cellulose from plants (Czaja et al.,
2007). Cellulose and BC also constructed by glucose
unit that linked through 1,4-β--glycoside, this
resulted in the highest crystallinity degree of
cellulose and BC, and it cannot soluble in water and
other common solutions. The different between
these two celluloses is the fiber size. Generally, BC
has a nanosized fiber but in cellulose-plants, the
most common sized of fiber is micro-sized. The
other advantage of BC is mostly free from
impurities, i.e. pectin and lignin.
Cellulose is found in agricultural waste,
including 58% rice husk, 56.86% sago bark, corn
cob 44.9%, 40-45% hardwood, 38-49% softwood,
oil palm empty fruit bunches 36 - 42%, esparto 33-
38%, bagasse 32-44%, wheat straw 29-37%, rice
straw 28-36% and bamboo around 26-43% (Ito et
al., 2007).
Cellulose-based hydrogels can be obtained via
either physical or chemical stabilization of aqueous
solutions of cellulosics (Chang et al., 2008).
Additional natural and/or synthetic polymers might
be combined with cellulose to obtain composite
hydrogels with specific properties (Sarkar, 1979).
One polymeric material that currently famous for
multipurpose is hydrogel which can be synthesis
from natural and synthetic polymer, e.g. cellulose,
chitosan, alginate, etc. Hydrogel as the multipurpose
material has been utilized for medical, cosmetic,
wound healing, agriculture, etc. Hydrogel can be
synthesized through several techniques, one of them
is semi-IPN. This technique has a lot of advantages,
especially to the chemical and physical properties of
the obtained hydrogel. The objective of the current
study was to determine the physical properties of the
hydrogel and learn about its chemical structure
through FT-IR data also the morphological from
SEM images.
Hydrogels can be produced through the
Interpenetrating Polymer Network (IPN) technique.
Interpenetrating Polymer Network (IPN) is a
polymerization system that is made simultaneously
Rizky, P., Tamrin, . and Marpongahtun, .
Synthesis of Semi Interpenetrating Polymer Network’s Hydrogel from Bacterial Cellulose.
DOI: 10.5220/0010132800002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 37-41
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
37

and sequentially to produce overlapping networks.
IPN is made through crosslinked polymeric
materials that are synthesized by condensation,
addition or propagation using several monomers,
then the resulting polymer is polymerized and
crosslinked (Banerjeer et al., 2010).
The crosslinker used in this study is N, N′-
methylene bisacrylamide (MBA) which reacts with
carboxyl functional groups in the polymer chain to
form a polymer network. The ability of polymers to
absorb water is very dependent on the degree of
crosslinking (Garner et al., 1997).
Acrylic Acid (AA) is a type of hydrophilic
monomer which is the raw material for the
manufacture of acrylic polyacetylenes (PAA)
polymers. The chemical structure of the acrylic
acrylic (PAA) has ionizable -COOH group units.
This polymer chain can be crosslinked to the -OH
group. By progress in the development of research
and technology, in recent years research relating to
acrylic acid polymers (PAA) is being intensively
developed as a base material for new biomaterials
(Billmeyer, 1984).
The combination of biopolymers and synthetic
polymers will produce superior hydrogels. Cellulose
is one of the basic ingredients of hydrogel which is
widely used as a raw material for wound dressing,
inexpensive, biodegradable, and biocompatible
(Sannino et al., 2009). Cellulose can also be
produced by microorganisms, bacterial cellulose
(BC) which is purer than cellulose derived from
plants because it is free of lignin, hemicellulose, and
other biogenic products (Brown, 2007).
2 MATERIALS AND METHODS
2.1 Materials
The chemical in this study were analytical grade, i.e.
sodium hydroxide (Merck), acrylic acid (Sigma
Aldrich), N,N’-methylenebisacrylamide (Merck),
potassium persulfate (Merck), and Polyethylene
glycol 1000 (Merck). Bacteria cellulose in this study
was obtained by culturing A. xylinum in the coconut
water medium (Gea et al., 2011).
2.2 Synthesis of Semi-IPN’s Hydrogel
About 200 mg of bacteria cellulose powder was
suspended in 100 mL of distillate water that
contained PEG and NaOH 3:4. The suspension was
placed in freezer at -5
o
C for 24 h. The frozen
suspension was melted and homogenized using
magnetic stirrer at room temperature for 2 h, as the
result the transparent solution was obtained. About 5
mL of the transparent solution was reacted with 760
mg of AA, 40 mg of MBA, and 54 mg of KPS.
These mixtures were stirred at 60
o
C for 15 min until
all components was homogenous. At the end of
polymerization, the transparent hydrogel was
washed with distillate water until the neutral pH was
reached. The obtained hydrogel was dried in oven
and kept in desiccator. This procedure was repeated
for the other composition of BC (400 mg; 600 mg;
800 mg and 1000 mg).
2.3 Characterization
The obtained hydrogel was characterized using FT-
IR (Bruker Opus Alpha 7.5) and SEM (Quorum
Model Q150R ES). The physical property of
hydrogel also characterized, i.e. swelling degree and
crosslinked percentage.
Swelling degree
Five sample replications were dried in oven at
60
o
C until the constant weight was obtained. These
samples were soaked in water for 24 h. The swollen
samples were separated from the solvent and
weighed (S. K. Bajpai & Swarnkar, 2014),. The
swelling degree of these samples were determined
using the equation (1) below:
Rasio Swelling (%) = (W
s
-W
d
)/W
d
× 100% (1)
Where W
s
was the weight of swollen sample and W
d
was the weight of dried sample.
Crosslinked degree
Five sample replications were dried in oven at 60
o
C
until the constant weight was obtained. These
sample were soaked in chloroform for 24 h and then
dried at 60
o
C for 3 h. The dried hydrogel was
weighed (S. K. Bajpai & Swarnkar, 2014), and the
crosslinked degree was determined using the
equation (2) below:
Crosslinked degree (%) = W
a
/W
b
× 100 % (2)
where W
a
was the dried weight of hydrogel before
soaked in chloroform and W
b
dried weight of
hydrogel after soaked in chloroform.
3 RESULTS AND DISCUSSIONS
3.1 Synthesis of Semi-IPN’s Hydrogel
The semi-IPN’s hydrogel (Fig.2) was prepared from
BC in the combination of poly-AA. As in method,
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
38
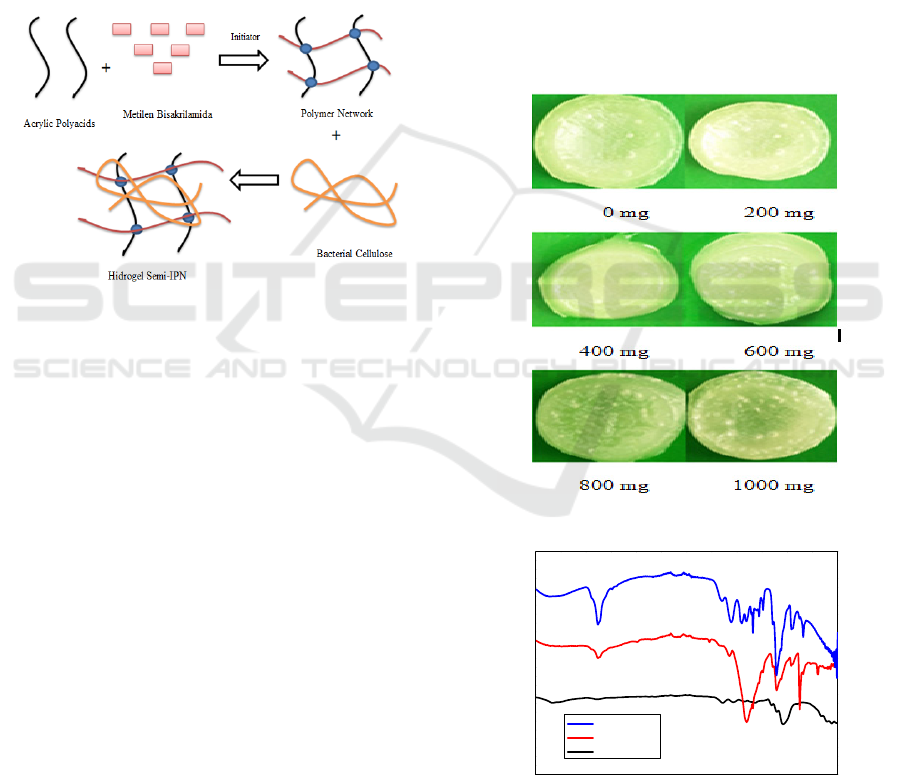
this hydrogel was prepared by varying the mass of
BC. The obtained hydrogel was prepared using free
radical polymerization technique, the initiator was
KPS and the crosslinker was MBA. The obtained
semi-IPN’s hydrogel was reacted in the alkaline
condition by the using a solvent system of
PEG/NaOH.
The chemical structure of semi-IPN’s hydrogel
was constructed by the crosslinked poly-AA with
MBA (crosslinked network of poly-AA). In other
hand, the BC in the hydrogel system was overlapped
with the crosslinked network of poly-AA.
Illustration of making semi-IPN hydrogel and
hydrogel can be seen in Fig.1.
Figure 1: Illustration of Semi-IPN Hydrogel
Manufacturing.
3.2 Fourier Transform Infrared
Spectroscopy (FTIR) Analysis
FTIR spectra of BC, poly-AA, and semi-IPN’s
hydrogel can be seen in Fig.3.
In the FTIR spectrum of bacterial cellulose
(Figure 1), there is an absorption peak at 3336 cm
-1
indicating the
presence of hydroxyl (O-H)
stretching. Also, there is an absorption peak of 2891
cm
-1
indicating the presence of -C-H bonds. At the
peak of the absorption of 1424 cm
-1
is the vibration
vibrations of -CH
2
. There is also an absorption peak
of 1157 cm
-1
where the spectrum shows uptake of -
C-O groups originating from bonds between carbon
atoms and hydroxyl groups in bacterial cellulose
(Suo et al., 2007).
FT-IR spectra of poly-AA and semi-IPN showed
a similar band with the spectrum of BC, but only
different in their intensities. A significance different
can be observed near 1600-1650 cm
-1
that assigned
as secondary amine group of N,N’-
methylenebisacrylamide (MBA). Also, the most
dominant band of this kind of sample can be
observed at 3000-3600 cm
-1
that confirmed the
presence of hydroxyl group from BC and acrylic
acid. The presence of secondary amine also can be
observed near 715 – 874 cm
-1.
Based on Fig.3, the shape and absorption band of
600 mg semi-IPN semi-IPN hydrogel did not have a
significant difference with the infrared absorption of
the hydrogel blank. This proves that the cross-
linking process between AA monomers (forming
polyacrylic acid) and the MBA cross-linker has been
formed. The existence of SB is proven to be able to
provide a physical bonding effect characterized by
the formation of semi-IPN hydrogels without
changing the shape of the absorption band on the
hydrogel made. The description of functional groups
that were successfully observed by FTIR
spectrophotometer between hydrogel blanks and 600
mg semi-IPN hydrogels did not differ much only by
shifting wave numbers (Sannino et al., 2009).
Figure 2: The Obtained Semi-IPN’s Hydrogel.
3500 3000 2500 2000 1500 1000 500
Hidrogel 600 mg
Blanko
Bacterial Cellulose
Transmittance (%)
Wavenumber (cm
-1
)
Figure 3: FTIR spectra of (a) BC, (b) poly-AA, and (c)
semi-IPN with 600 mg of BC.
Synthesis of Semi Interpenetrating Polymer Network’s Hydrogel from Bacterial Cellulose
39
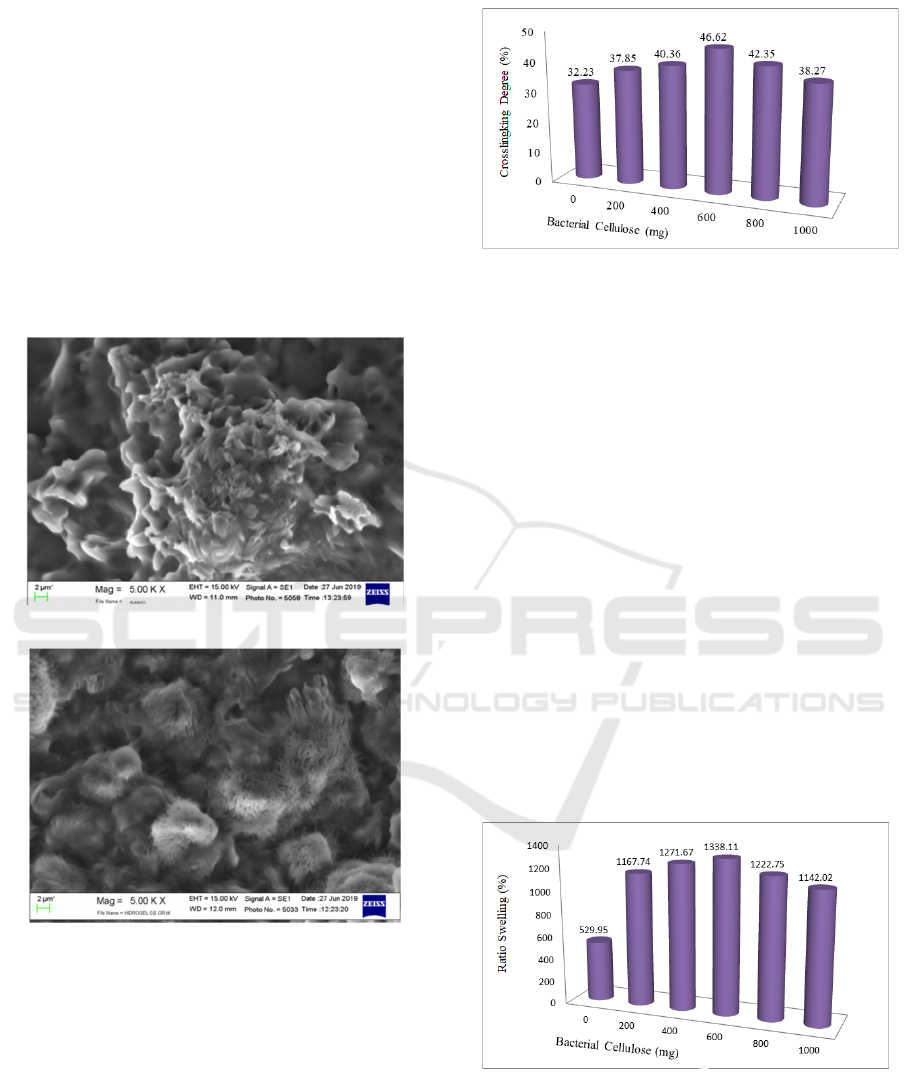
3.3 Scanning Electron Microscopy
(SEM) Analysis
The morphological analysis using SEM was
performed to observe the surface characteristic of
the obtained semi-IPN’s hydrogel. Fig. 4 (a) the
obtained hydrogel poly-AA formed an agglomerate.
Hebeish et al. (2014) in his research explained that
the formation of agglomerate was twisting effect, an
indication of interaction. But a unique result was
found in Fig 4 (b), small pores were observed on the
hydrogel surface. The presence of this pores in the
semi-IPN’s hydrogel can enhance the diffusion and
swelling degree of hydrogel (Astrini et al., 2016).
a
b
Figure 4: The morphological of hydrogel (a) poly AA and
(b) semi-IPN.
3.4 Crosslinked Degree Analysis
The result of crosslinked degree is exhibited in Fig
5.
Figure 5: The crosslinked degree of semi-IPN’s hydrogel.
The crosslinked degree with the maximum is
observed in the addition of 600 mg of BC. This can
be assumed as the formation of a long polymer chain
and was linkage in the specific positions. But the
decrease of crosslinked degree observed in the
addition of 800 mg and 1000 mg of BC. This can be
assumed at this amount of BC the elasticity modulus
of semi-IPN was limited as the van der Waals and
hydrogen bonding interactions between BC and AA.
This decrease is caused by the occurrence of
equilibrium so that the addition of SB can reduce
mechanical properties due to irregular polymer
chains (Dragan et al., 2012).
3.5 Swelling Degree Analysis
The ability of hydrogel for absorbing water was
determined using swelling degree. Hydrogel can be
swelling, has a transparent and smooth surface, and
flexible (Okay & Sariisik, 2000). The swelling
degree of semi-IPN’s hydrogel can be seen in Fig.6.
Figure 6: Swelling Degree Of Semi-IPN’s Hydrogel.
Based on the Fig.5, the swelling degree increase
with the increase of BC in the hydrogel system. But
the value decrease when the addition of BC is more
than 0.6 g. For the first case, with the increase of BC
in the hydrogel system it will have a direct impact to
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
40

the number of hydroxyl group. The increase of this
hydroxyl group will influence the hydrophilicity of
hydrogel. But in the second case, the increase of
hydroxyl group may disturb the equilibrium of
water, as the impact the water diffusion become low
(S. K. Bajpai & Swarnkar, 2014). The other reason
of the decrease of swelling degree at the addition of
BC of 0.8 and 1.0 g can be caused the ratio between
MBA-poly AA and BC was not balanced. It can be
assumed, as the impact there are some of BC that
will not interact with MBA-poly AA through van
der Waals and hydrogen bonding interaction (A. K.
Bajpai & Giri, 2003).
4 CONCLUSIONS
Semi-IPN’s hydrogel has been successfully
synthesis using BC, AA and MBA through free
radical polymerization. The maximum value of
crosslink degree and swelling degree was found at
600 mg, with the value of 46,62% and 1338%,
respectively. The FT-IR spectra confirmed the
crosslinked of MBA to AA was found at 1555 cm
-1
.
The morphological surface of semi-IPN’s hydrogel
showed a rough and dense surface.
ACKNOWLEDGEMENTS
The authors thank the Polytechnic Industrial
Chemical Technology, Department of Chemistry,
Universitas Sumatera Utara, Medan for their support
in the use of laboratories, and do not forget my
supervisor who has provided the benefits of
guidance and advice in conducting this research.
REFERENCES
Astrini, N., Anah, L., & Haryono, A. (2016). Pengaruh
Metilen Bisakrilamid (MBA) pada Pembuatan
Superabsorben Hidrogel Berbasis Selulosa terhadap
Sifat Penyerapan Air. Jurnal Kimia Dan Kemasan,
38(1), 15–20.
Bajpai, A. K., & Giri, A. (2003). Water Sorption
Behaviour Of Highly Swelling (Carboxy
Methylcellulose-g-Polyacrylamide) Hydrogels And
Release Of Potassium Nitrate As Agrochemical.
Carbohydrate Polymers, 53(3), 271–279.
Bajpai, S. K., & Swarnkar, M. P. (2014). New Semi-IPN
Hydrogels Based On Cellulose For Biomedical
Application. Journal of Polymers, 2014, 1–12.
Banerjeer, S., Ray, S., Maiti, S., Sen, K., Bhattacharyya,
U., Kaity, S., & Ghosh, A. (2010). Interpenetrating
Polymer Network (IPN): A Novel Biomaterial.
International Journal of Applied Pharmaceutics, 2,
28–34.
Billmeyer, F. (1984). Textbook Of Polymer Science
(Third). John Wiley and Sons.
Brown, E. E. (2007). Bacterial cellulose/thermoplastic
polymer nanocomposites. Department of Chemical
Engineering, Washington State University.
Chang, C., Lue, A., & Zhang, L. (2008). Effects of
crosslinking methods on structure and Properties Of
Cellulose/PVA Hydrogels. Macromol. Chem. Phys.,
209(12), 1266–1273.
Czaja, W. K., Young, D. J., Kawecki, M., & Brown, R. M.
(2007). The future prospects of microbial cellulose in
biomedical applications. Biomacromolecules, 8(1), 1–
12.
Dragan, E. S., Perju, M. M., & Dinu, M. V. (2012).
Preparation and Characterization of IPN Composite
Hydrogels Based on Polyacrylamide and Chitosan and
Their Interaction with Ionic Dyes. Carbohydrate
Polymers, 88, 270–281.
Garner, C. M., Nething, M., & Nguyen, P. (1997).
Synthesis Of A Superabsorbent Polymer. J. Chem.
Educ., 74, 95–99.
Gea, S., Reynolds, C. T., Roohpour, N., Wirjosentono, B.,
Soykeabkaew, N., Bilotti, E., & Peijs, T. (2011).
Investigation Into The Structural , Morphological ,
Mechanical and Thermal Behaviour of Bacterial
Cellulose After A Two-Step Purification Process.
Bioresource Technology, 102(19), 9105–9110.
Hebeish, A., Farag, S., Sharaf, S., & Shaheen, T. I. (2014).
Thermal Responsive Hydrogels Based on Semi
Interpenetrating Network of Poly(NIPAm) and
Cellulose Nanowhiskers. Carbohydrate Polymers,
102, 159–166.
Ito, T., Yeo, Y., Highley, C. B., Bellas, E., Benitez, C. A.,
& Kohane, D. S. (2007). The prevention of peritoneal
adhesions by in situ cross-linking hydrogels of
hyaluronic acid and cellulose derivatives.
Biomaterials
, 28(6), 975–983.
Okay, O., & Sariisik, S. B. (2000). Swelling Behavior of
Poly (Acrylamide-co-Sodium Acrylate) Hydrogels in
Aqueous Salt Solutions: Theory Versus Experiments.
Eur. Polym. J., 36, 393–399.
Ross, P., Mayer, R., & Benziman, M. (1991). Cellulose
Biosynthesis and Function in Bacteria. Microbiol.
Rev., 5(1), 35–58.
Sannino, A., Demitri, C., & Madaghiele, M. (2009).
Biodegradable Cellulose-based Hydrogels: Design and
Applications. Materials (Basel), 2(2), 353–373.
Sarkar, N. (1979). Thermal gelation properties of methyl
and hydroxypropyl methylcellulose. J. Appl. Polym.
Sci., 24(4), 1073–1087.
Suo, A., Qian, J., Yao, Y., & Zhang, W. (2007). Synthesis
and Properties of Carboxymethyl Cellulose- graft-
Poly(acrylic acid-co-acrylamide) as a Novel Cellulose-
Based Superabsorbent. J. Appl. Polym. Sci., 103,
1382–1388.
Synthesis of Semi Interpenetrating Polymer Network’s Hydrogel from Bacterial Cellulose
41
