
The Effect of Hydrochloric Acid (HCL) Concentration on the Quality
of Gourami Bone Gelatin (Ospheronemus Gouramy Lac)
Anggita Rahmi Hafsari
1
, Diah Rosmiati
1
, Dindin Jamaludiin
2
1
Departement of Biology, Faculty of Science and Technology State Islamic University (UIN) Sunan Gunung Djati Bandung,
JL.AH. Nasution NO 105 Bandung, 40614, Indonesia
2
Department of Islamic Education and Teaching, faculty of Teaching and Education State Islamic University (UIN) Sunan
Gunung Djati Bandung JL.AH. Nasution NO 105 Bandung, 4061, Indonesia
Keywords: Gelatin, Fish Bone, Halal Gelatin
Abstract: Gelatin is commonly made of the skin and bones of cattles or pigs. This causes problems in the society both
in terms of the halal status and health. Fish bone (Osphronemus gouramy Lac) can be used for producing
gelatin because it contains collagen protein. The purpose of this study was to produce good gelatin with
different concentrations and to identify the physical characteristics of gelatin from the bones of gouramy fish
at several concentrations. The treatment performed in this study used HCl was used with 48 hours immersion
period and concentration of 1% - 5%. The value of rendement in this study ranged from 8.05% - 12.44%, the
viscosity value ranged from 4.7 to 6.1 cP, the gel strength ranged from 71.90 to 90.61 bloom, the water content
ranged from 9% - 12%, the grain ranged from 2.18% - 3.78%, pH levels ranged from 4.64% - 4.85% and the
protein content ranged from 59.68% - 70.24%. Based on these results, it can be concluded that the different
concentrations of acids significantly affect the value of gelatin produced. The best concentration is at 5%. At
this concentration, the gelatin produced has the best physical and chemical properties compared to those
produced with other concentrations.
1 INTRODUCTION
Gelatin is derived proteins from collagen that in
denaturing because of termo-hidrolisis and to
transform termo-reversible between sol and gel (Cho,
2004). Gelatin is a protein conversion is soluble in
water (Sobral, 2001).
Besides food industries, gelatin is also used in
non-food industries such as pharmaceutical industry,
photography, cosmetics, and paper industries. Gelatin
can be used in the forms of capsules, tablets and
pastilles, gelatin sponge, surgical powder, medical
research, plasma expander, and microencapsulation
in the pharmaceutical field.
Gelatin available in the market is mostly made
from the bones of pigs or other mammals such as
cows. In the teaching of Islam, gelatin from pigs is
considered haram (forbidden to consume). This
relates to the Islamic Shari'a law, which requires its
followers to consume only halal food. On the other
hand, the consumption of beef gelatin is often
worrying as cattles are often infected by diseases such
as anthrax and mad cow. In addition, often, in the
process of slaughtering, the name of Allah was not
mentioned and the cut was not made on the jugular
vein of cows, making the beef unlawful. The Quran
describes the prohibition of eating unclean foods, as
follows: Prohibited to you are dead animals, blood,
the flesh of swine, and that which has been dedicated
to other than Allah, and [those animals] killed by
strangling or by a violent blow or by a head-long fall
or by the goring of horns, and those from which a wild
animal has eaten, except what you [are able to]
slaughter [before its death], and those which are
sacrificed on stone altars, and [prohibited is] that you
seek decision through divining arrows. That is grave
disobedience. (Al-Maidah verse: 3).The importance
of halal foods is regulated in Islam as stated in the
Qur'an: "O mankind, eat from whatever is on earth
[that is] lawful and good and do not follow the
footsteps of Satan. Indeed, he is to you a clear
enemy." (Al-Baqarah 168). Besides, Rasulullah
PBUH also said: "Whoever takes a bite of forbidden
goods, then the prayer will not be accepted for forty
days" (HR. Abu Daud).
Rahmi Hafsari, A., Rosmiati, D. and Jamaludin, D.
The Effect of Hydrochloric Acid (HCL) Concentration on the Quality of Gourami Bone Gelatin (Ospheronemus Gouramy Lac).
DOI: 10.5220/0009947329832989
In Proceedings of the 1st International Conference on Recent Innovations (ICRI 2018), pages 2983-2989
ISBN: 978-989-758-458-9
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
2983

Therefore, obtaining alternative raw materials
for producing halal gelatin is necessary. Gourami
bones are one of the materials which can be used to
produce halal gelatin. Further, gelatin from fish bones
is safer in terms of health because it is not made from
infected materials that can affect the health of
consumers. So, it is expected that the gelatin has a
high quality and meets the standards of commercial
gelatin.
The purpose of this study was to determine the
highest concentration of HCl to generate the best
gelatin with different concentrations and to determine
the effect of HCl concentration on the physical and
chemical characteristics of Gourami bone gelatin.
2 MATERIALS AND METHODS
2.1 Equipments
The equipment used in this study is divided into two:
the equipment used in the manufacture of gelatin is an
analytical balance, beaker, small spoons, measuring
cups, glasses, water bath, an evaporator, a pH meter,
viscometer, Kjeldhal pumpkin.
2.2 Materials
The raw materials used are Gourami bon dry waste as
much as 2 kg, 6 M HC1 distilled water, filter paper
and aluminum foil
2.3 Experimental Design
This study is divided into two phases. The first phase
of the preliminary test, which is the process of making
gelatin from the bones of Gourami (Ospheronemus
Gourami) with a concentration of 2%, 4%, 6%, 8%,
10% and analyzing physical properties that yield. The
second stage of research the manufacture of gelatin,
namely the manufacture of gelatin concentrations that
are the 1%, 2%, 3%, 4%, after the gelatin was chosen
to analyze physical and chemical properties of gelatin
that is yield, viscosity, gel strength, analysis of the
degree of acidity (pH ), moisture content, ash content,
and protein.
2.4 Research Stages
2.4.1 Gelatin Extraction
Bone degreasing or boiled for 20 minutes at 70 ° C
with bone and distilled water ratio 1: 2 (b: v) and then
dried in the sun until bone dry. Degreasing the bones
that have been soaked in a solution of HCI at a
concentration of 1%, 2%, 3%, 4% and 5% for 24
hours (treatment A). The bones that have been soaked
in an acid solution is called ossein and separated and
then filtered. Ossein then neutralized with
water. Ossein the neutral buffer is inserted into the
glass beaker and add distilled water, ossein
comparison with distilled water is 1: 2 (w / w). After
it is extracted with a water bath at a temperature of 90
° C for 7 hours. Then filtered through Whatman filter
paper No. 40. Distillate concentrated by
evaporator. Gelatin solution obtained was still in a
state of liquid. The gelatin solution should be
concentrated. Concentration is done with the
evaporator, until a concentration to 25-30%, the
concentration temperature is 800C, while the time it
takes ± 5 hours (Handoko et al, 2011).
2.5 Research Analysis
2.5.1 Rendemen
Randemen obtained from the weight ratio of dry flour
weight gelatin produced with fresh ingredients (the
bone that has been washed). by AOAC, 1995:
Rendemen =
× 100%
2.5.2 Gel Strength
Gel strength is done objectively by using the tool
Rheoner RE3305. The level of gel strength expressed
in units gf/cm2, which means the amount of
compressive force to break up the deformation of the
product. (British Standard 757, 1975).
2.5.3 Viscosity
The water content of the gelatin is determined by
providing the chemicals as much as 75-100 ml in the
samples estimated to contain as much as 2-5 ml water,
then heated to boiling for 1 hour. Water vapor and
chemicals are condensed and collected in the
reservoir tube. Tools used as a container for include
Strak tube-Dean and Sterling-Bidwell (Nancy, 2013).
%water content =
100
2.5.4 Ash Content
The ash content is determined by weighing the
sample ± 2 grams ago, then put into porcelain dish
until it is heated in a charcoal bath. Samples were
evaporated water is put into the furnace temperature
to 600 0C. The evaporation process until all material
ICRI 2018 - International Conference Recent Innovation
2984
changes color to gray, then the sample is weighed
(AOAC, 1995).
% Ash Content =
x 100%
2.5.5 Acidity (pH)
A total of 0.2 gram sample is weighed and dispersed
into 20 ml of distilled water at a temperature of 80 °
C. The samples were homogenized with a magnetic
stirrer, and then measured the degree of acidity at
room temperature with a pH meter (British Standard
757, 1975)
2.5.6 Protein Level
A total of ± 0.25 gram dry sample, placed in a 100 ml
Kjeldahl flask and added 0.25 grams of selenium and
3 ml of concentrated H2SO4. Then proceed with the
process for 1 hour until a clear solution. Once cool
add 50 ml of distilled water and 20 ml of 40% NaOH,
and then distilled. Distilled accommodated in a
Erlenmeyer flask containing a mixture of 10 ml of
H3BO3 2% and 2 drops of indicator Brom Crsol
Green Methyl Red pink. Once the volume of
reservoirs (distillate) to 10 ml and the bluish-green,
distillation was stopped and distilled titrated with
0.0235 N HCl until pink. The same treatment was
also carried out on the blank. With this method
obtained the total nitrogen content calculated using
the formula (AOAC, 1995):
% Protein Level =
..
2.6 Data Analysis
Data were analyzed with SPSS 20.0 by ANOVA. If
the results of the analysis significantly different then
tested further using Duncan (Gaspersz 1994).
3 RESULTS AND DISCUSSIONS
3.1 Analysis of Physical Properties of
Gelatin
3.1.1 Rendemen
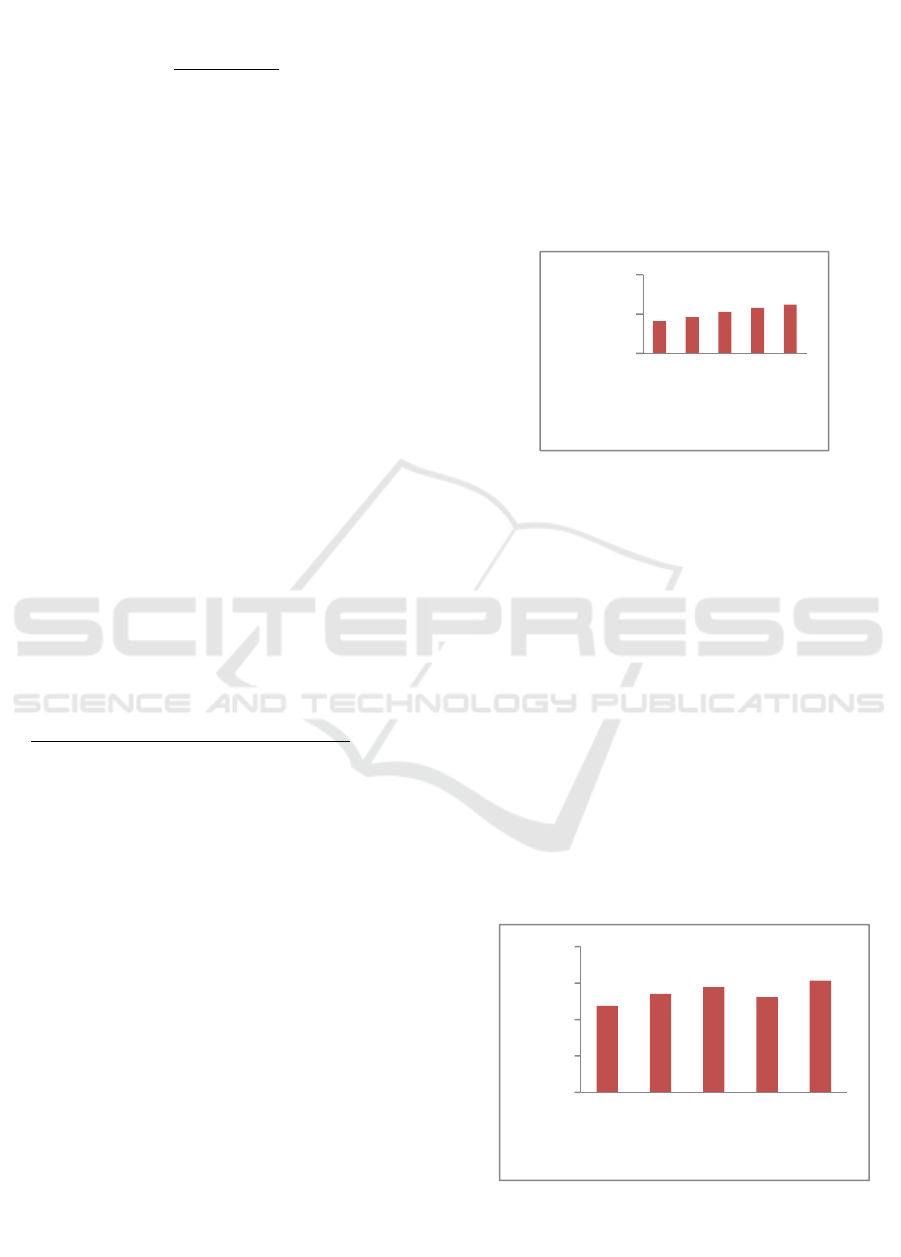
Based on Figure.1 The yield values obtained in
gelatin by treatment with HCl 5%, ie 12.44%, while
the value of the smallest yield generated 1% HCl
concentration of 8.05%. According to Mulyani et al
(2013), HCl has a bigger and stronger ability to
degrade CaCO3 minerals of calcium is in the bones
of Gourami compared with a base. Conversion of
collagen into gelatin can be affected by temperature,
heating time, and pH. In attachment.5 the values
ranged on average from 8.16 to 12.44%. The size of
the yield value is influenced by the concentration of
HCl is different. The yield shows the effectiveness of
the methods used in a study, especially about
optimalitasnya in producing a product. The higher the
yield value indicates the treatment is applied to the
research more effective (Miwada and Simpen, 2007)
Figure 1 The value of bone Gourami rendemen gelatin.
Description: Different letters indicate a significant
difference at a significant level α = 0,05
3.1.2 Viscosity
Viscosity is the power flow of molecules in a solution
either in water, a simple organic liquid and an
aqueous suspension (Fever, 1989). The average value
of Gourami viscosity bone gelatin is 4.7 to 6.1 cP
(Figure.2). This research viscosity grades according
to standards set by GMIA in 2012 is between 1.5 to
7.5 cP. According to Setiawati (2009), viscosity or
viscosity of the gelatin solution is closely related to
the water content of dry gelatin. The lower the water
content of dry gelatin then its ability to bind water (to
form a gel) will be higher. The more the amount of
water bound by the gelatin will become increasingly
viscous gel, which directly affect the higher the
viscosity measured value.
Figure 2 Viscosity Values Gourami Bone Gelatin.
Description: Different letters indicate a significant
difference at a significant level α = 0.05
a
b
c
d
e
0
10
20
12345
Rendemen …
HCl Concentratio
n
a
c
d
b
e
0
2
4
6
8
12345
Viscosity cP
HCl Concentration (%)
The Effect of Hydrochloric Acid (HCL) Concentration on the Quality of Gourami Bone Gelatin (Ospheronemus Gouramy Lac)
2985
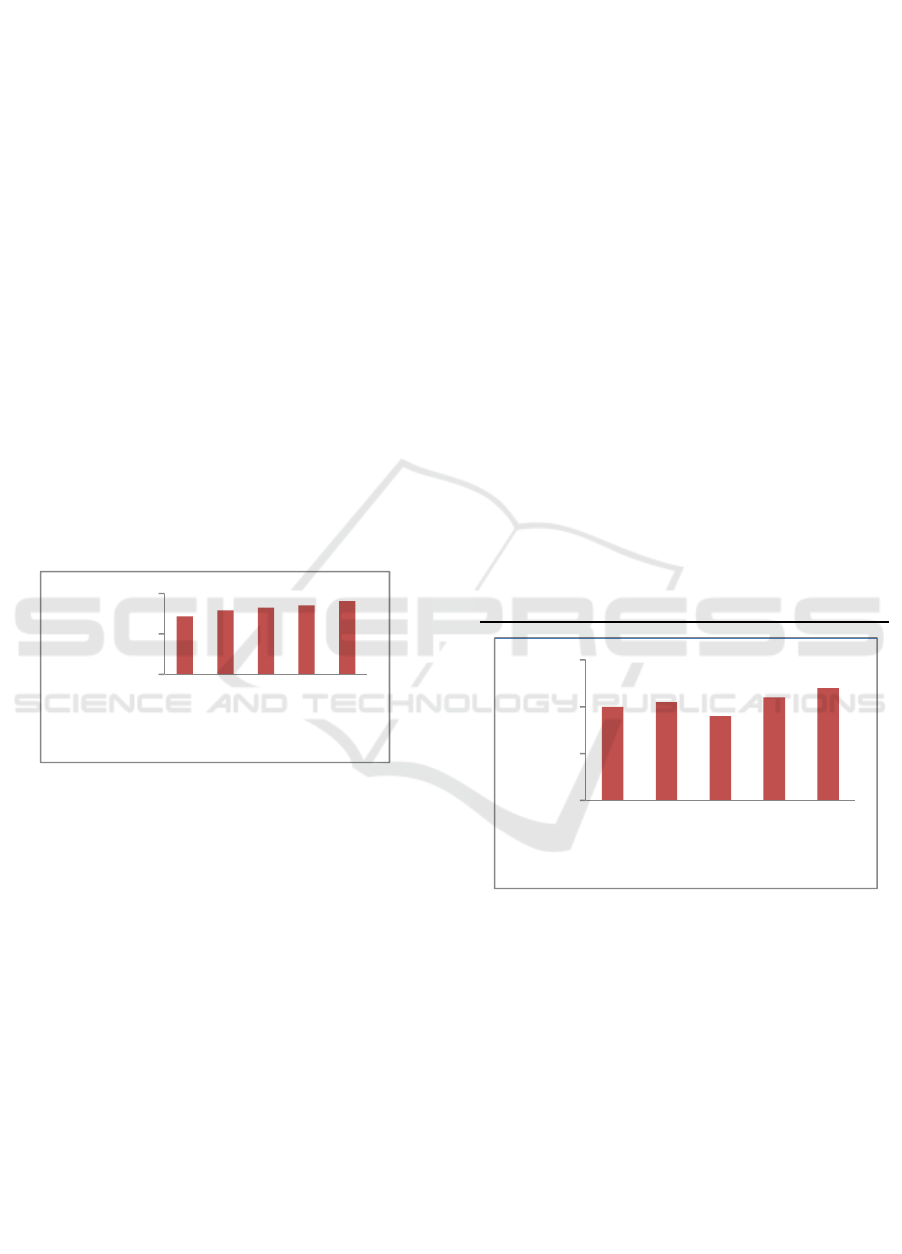
3.1.3 Gel Strength
Gel strength is important in knowing the best
determination, because one of the important
properties of gelatin is able to transform the liquid
into a gel which is reversible. Ability is what causes
the gelatin very wide use, both in the field of food and
non-food (Harris, 2008).
Based on advanced test Duncan showed that
the treatment with using a 5% concentration showed
the highest gel strength, which amounted to 90.61
bloom, while for the results of the gel strength
smallest one is at a concentration of 1% at 71.90
bloom (Figure.3). The use of different concentrations
significant effect, this is caused by collagen with acid
hydrolysis process went quite well with increasing
concentrations and the heating process that would
damage the gelatin to form a gel structure. According
Astawan (2002), high gel strength associated with
long-chain amino acids, which long chains of amino
acids that will produce a large gel strength. Optimal
hydrolysis process will result in a long chain of amino
acids, and at the time of conversion of collagen into
gelatin will produce a high gel strength.
Figure 3: Value of Gelatin Gourami Bone Strengt.
Description: different letters indicate a significant
difference at a significant level α = 0.05
3.2 Gelatin Chemical Properties
Analysis
3.2.1 Water Content
Water is an essential ingredients in a food. Water can
be either intracellular or extracellular components of
a product (deMan1989). Water in food ingredients
will determine the acceptability, freshness and
durability of the material. Water can also affect the
appearance, texture, taste, and quality of foodstuffs
(Winarno, 1992).
Differences in water content varying
allegedly due to the use of materials that are not
evenly distributed between the hard bone and
cartilage in each treatment thus affecting the results
of the water content of the gelatin bones of Gourami,
besides the difference in the water content of the
gelatin is affected by the drying process each sempel
for every sempel the concentration difference has a
drying time is different.The statement was supported
by Rev. et al (2013) that for every sample requires a
different time to be a dry sample.
The average value of the water content of
Gourami bone gelatin ranging between 9% -
12%. The highest water content is at 5% HCl
concentration of 12%, while the smallest water
content is at a concentration of 3% by 9%
(Figure.4). Differences in water content was
allegedly influenced by different raw materials in
each sample. Water content of the fifth HCl
concentration of gelatin still meet the quality
standards of ISO No. 53 of 1995 which is up to 16%
and the standard of JECFA in 2003 a maximum of
18%.
According to deMan (1997) gelatin moisture
content affects the shelf life of a product, because it is
closely related to metabolic activity that occurred
during the gelatin is stored. The role of water in food
is one of the factors that influence the metabolic
activity such as enzyme activity, microbial activity
and chemical activity, namely the occurrence of
rancidity and non-enzymatic reactions giving rise to
changes in organoleptic properties and quality values.
Figure 4: Water content Gelatin Gourami Bone.
Description: Different letters indicate a significant
difference at a significant level α = 0.05
3.2.2 Ash Content
Ash is an organic substance that is not burned in the
combustion process of organic substances. These
substances include sodium, chlorine, calcium,
phosphorus, magnesium, and sulfur (Winarno
1992). Ash content value of foodstuffs showed a large
amount of minerals contained in the food of a
substance (Apriyantono 1989).
a
b
cde
0
50
100
12345
Gel Strength
(bloom)
HCl Concentration (%)
ab
ab
a
ab
b
0
5
10
15
12345
Waterr Content (%)
HCl Concentration (%)
ICRI 2018 - International Conference Recent Innovation
2986

Figure 5: Levels of Ash Gelatin Gourami Bone .
Description: Different letters indicate a significant
difference at a significant lvel α = 0.05
Duncan advanced test showed that the concentration
of HCl gives a significantly different effect (P ≤ 0.05)
with a concentration of 3%, 4% and 5%, but not
significantly different (P ≥ 0.05) with a concentration
of 1% and 2% (Figure.5). Decreased calcium in the
ossein is what causes the reduction of the value of its
own ash content. Revelation et al (2013) states that
the use of different acid concentrations have a
significant influence on the ash content of
gelatin. Marked with the rate of decline of the gelatin
ash content along with the increasing concentration of
acid used solvents. Allegedly higher acid
concentrations can dissolve bone mineral in large
quantities by the leaching process. According to
Mulyani et al (2013), the use of HCl in the immersion
process will react with the calcium phosphate
bone. This will produce a soluble calcium salt and
bones become soft, because HCl has bigger and
stronger ability to degrade CaCO3 minerals of
calcium is in the bones of Gourami compared with the
use of bases
Value ash content contained in a product
indicates the level of purity of the product.The level
of purity is affected by the composition and mineral
content. Abu contained in gelatin derived from salts
or minerals in the bones of fish used (Ratri, 1998).
3.2.3 Acidity (pH)
The pH value of the degree of acidity gelatin is gelatin
which is one of the important parameters in quality
standards gelatin. pH gelatin based gelatin quality in
general is expected to approach a neutral pH (Heidi,
2006).
Figure 6 pH of Gelatin Bone Gourami . Description:
Different letters indicate significant differences at the level
of α = 0.05
In this study it was found that the higher the
concentration of HCl pH value lower. The low pH
value is due to the use of strong acid HCl. Allegedly
in the event of collagen development time soaking
with HCl, much residual unreacted HCl absorbed in
the collagen which expands and caught in the
collagen fibril network, making it difficult neutralized
when washing that eventually brought current
extraction processes that affect the level of acidity. In
Appendix 5 the average pH value in Gourami bone
gelatin ranged from 4.64 to 4.85%. The highest pH
value is at a concentration of 1% by 4.85%
(Figure.6). This is because the higher the
concentration is used it will be increasingly
concentrated acid and also the acid ossein which is
bound to be more and more. While the lowest value
contained at 5% concentration of 4.65%. Nurimala
(2004), stating Low pH values in this study because
it is still carrying over of residual HCl on fish bones
and carried the time of extraction, thus affecting the
value of acidity in the resulting gelatin. The pH value
of Gourami bone gelatin in the amount of 4.64 to
4.85%. According Hinterwaldner (1977) in Nofri et
al (2014) gelatin pH value relates to the process
used. Acid process tends to produce low pH, whereas
the alkaline process will have a tendency to produce
a high pH. Gelatin with a neutral pH is more preferred
that the neutralization process plays an important role
in the manufacture of gelatin.
3.2.4 Protein Level
Proteins are polymers of about 21 different amino
acids and peptides associated with the bond. Proteins
in the gelatin included in the group Scleroprotein
simple protein, because gelatin is obtained from the
hydrolysis of collagen. (De Man, 1989).
d
d
c
b
a
0
2
4
6
12345
Ash Content (%)
HCl Concentration (%)
d
d
c
b
a
4.4
4.6
4.8
5
12345
pH value (%)
HCl Concentration (%)
The Effect of Hydrochloric Acid (HCL) Concentration on the Quality of Gourami Bone Gelatin (Ospheronemus Gouramy Lac)
2987

Figure 7 Levels of Protein Gelatin Bone Gourami.
Description: notation different letters indicate a significant
difference at a significant level α = 0.05
Based on Figure 4.7 the value of the highest levels of
the protein present in a concentration of 5%
amounting to 70.24%, while the value of the lowest
levels of the protein present in a concentration of 1%
at 59.68%. Suspected high acid capable hydrolyze
collagen into gelatin. According to Rusli (2004), the
protein content of the gelatin is affected by the
immersion process of bone. Soaking process resulted
in the termination reaction of hydrogen bonds and the
opening of the coil structure of collagen occurring
optimally so that the amount of protein extracted into
many. Table 4.1 Based on the analysis of physical and
chemical properties of gelatin best results are at
concentrations of 5% with the result that much better
with the other concentration. This proves the higher
the concentration, the better the results of quality
gelatin. The value of physical and chemical
properties a concentration of 5% produces the highest
protein content with other concentrations of 70.24%,
with a yield of 12.44%, a value of 6.1 cP viscosity,
gel strength 90.61 bloom, the water content of 12%,
pH 4.64%, and ash content of 2.18%. According to
Martianingsih and Lukman (2010), the immersion
process using the acid solution will convert collagen
into a form suitable at the time of extraction in the
presence of H + ions from the acid solution with
collagen.
4 CONCLUSION
Acid concentrations varying significantly affect the
resulting gelatin. Based on this research the best
concentration present in concentrations of 5%. At the
concentration of gelatin values obtained with the
physical and chemical properties compared with
other concentrations. Treatment of acid
concentrations varying influence on the physical and
chemical properties of Gourami bone gelatin. The
results of the analysis of physical and chemical
properties showed significantly different results with
commercial gelatin and gelatin laboratory
standards. Based on the analysis of physical
properties that yield value of 8.05 to 12.44%, ranging
between 4.74 to 6.13 cP viscosity and gel strength of
71.90 to 90.61 bloom. As for the chemical analysis
that the ash content ranged from 3.78 to 2.18%, water
content ranging between 10-12%, the pH value
ranging between 4.64 to 4.85% for protein content
ranged from 59.68 to 70 , 24.
REFERENCES
Apriyanto A et al. 1989. Analisis Pangan. Bogor: IPB
press.
Astawan M, Aviana T. 2003. Pengaruh jenis larutan
perendaman serta metode pengeringan terhadap sifat
fisik, kimia, dan fungsional gelatin dari kulit cucut.
Jurnal Teknologi dan Industri Pangan. Vol 14 (1):7-12.
Association of Official Analytical Chemist (AOAC). 1995.
Official Methods Analysis Chemist. Vol IA.
Association of Official Anaytical Chemist, Inc.,
Washington.
British Standard 757. 1975. Sampling and Testing of
Gelatin.
deMan, John. M. 1997. Kimia Makanan Edisi Kedua.
Penerjemah Kosasih Padmawinata ITB. Bandung.
Gaspersz V. 1994. Metode Perancangan Percobaan. CV.
Armico. Bandung.
Handoko Tony, Sherly O dan Isabella S. 2011. Pengaruh
Jenis Dan Konsentrasi Asam, Temperatur Dan Waktu
Ekstraksi Terhadap Karakteristik Fish Glue Dari
Limbah Ikan Tenggiri. Jurusan Teknik Kimia.
Universitas Katolik Parahyangan
Haris M. 2008. Pemanfaatan Limbah Tulang Ikan Nila
(Oreochromis niloticus) Sebagai Gelatin Dan Pengaruh
Lama Penyimpanan Pada Suhu Ruang. (Skripsi).
Fakultas Perikanan Dan Ilmu Kelautan. Institut
Pertanian Bogor.
Heidi Wiratmaja. 2006. Perbaikan Nilai Tambah Limbah
Tulang Ikan Tuna (Thunnus sp) menjadi Gelatin Serta
Analisis Fisika-Kimia. (Skripsi). Fakultas Perikanan
Dan Ilmu Kelautan. IPB.
Hinterwaldner R. 1977. Technologi of gelatin manufacture.
Di dalam Nofri Sandria, Desmelati, Mery Sukmiwati.
2014. Studi Ekstraksi Gelatin Dari Mata Ikan Tuna
(Thunnus sp.). Jurnal Fakultas Perikanan dan Kelautan.
Universitas Riau.
Mulyani Tri, Sudaryati, Siska F Rahmawati. 2013.
Hidrolisis Gelatin Tulang Ikan Kakap Menggunakan
Larutan Asam. Tek.Pangan. FTI UPN. Veteran. Jatim
Miwada, IN. Sumerta dan IN. Simpen. 2007. Optimalisasi
potensi ceker ayam (shank) hasil limbah RPH melalui
metode ekstraksi termodifikasi untuk menghasilkan
gelatin (Jurnal). Jurusan Produksi Ternak, Fakultas
Peternakan, Universitas Udayana, Denpasar Jurusan
a
b
c
d
e
50
55
60
65
70
75
12345
Protein Level %
HCl Concentration (%)
ICRI 2018 - International Conference Recent Innovation
2988

Kimia, Fakultas MIPA, Universitas Udayana,
Denpasar.
Nurilmala M, 2004. Kajian potensi limbah tulang ikan keras
(Teleostei) sebagai sumber gelatin dan analisis
karakteristiknya [Tesis]. Bogor: Sekolah Pasca sarjana.
IPB.
Ratri Aryanti. 1998. Kajian Proses Produksi Gelatin Dari
Tulang Domba Menggunakan Proses Asam (Skripsi).
Fakultas Teknologi Pertanian. Institut Pertanian Bogor.
Bogor
Rusli A. 2004. Kajian proses ekstraksi gelatin dari kulit
ikan patin
(Pangasius hypopthalmus) segar [Tesis]. Bogor:
Sekolah Pasca sarjana. IPB.
Setiawati Hani Ima. 2009. Karakterisasi mutu fisika kimia
gelatin kulit ikan kakap merah (Lutjanus sp.) hasil
proses perlakuan asam. (Skripsi). Program Studi
Teknologi Hasil Perikanan Fakultas Perikanan dan
Ilmu Kelautan. Institut Pertanian Bogor.
Martiangsih, N. dan Lukaman A. 2010. Analisis Sifat
Kimia, Fisik, dan Termal Gelatin Dari Ekstraksi Kulit
Ikan Pari (Himantura gerradi) Melalui Variasi Jenis
Larutan Asam. Prosiding (Skripsi) Institut Teknologi
Sepuluh November. Surabaya. Hal 1-10.
Miwada, IN. Sumerta dan IN. Simpen. 2007. Optimalisasi
potensi ceker ayam (shank) hasil limbah RPH melalui
metode ekstraksi termodifikasi untuk menghasilkan
gelatin (Jurnal). Jurusan Produksi Ternak, Fakultas
Peternakan, Universitas Udayana, Denpasar Jurusan
Kimia, Fakultas MIPA, Universitas Udayana, Denpasar
Mulyani Tri, Sudaryati, Siska F Rahmawati. 2013.
Hidrolisis Gelatin Tulang Ikan Kakap Menggunakan
Larutan Asam. Tek.Pangan. FTI UPN. Veteran. Jatim.
Nancy Siti Djenar. 2013. Modul Penetapan Kadar Air.
Jurusan Teknik Kimia. Politeknik Negeri Bandung
Winarno FG 1992. Kimia Pangan dan Gizi. Jakarta: PT
Gramedia Pustaka Utama.
The Effect of Hydrochloric Acid (HCL) Concentration on the Quality of Gourami Bone Gelatin (Ospheronemus Gouramy Lac)
2989
