
Antihyperglycemic Effect and Glucose Tolerance of Ethanol Extract
the Rind of Jengkol (Pithecollobium jiringa Jack) in Diabetic Rats
Muhammad Yanis Musdja
1
, Weldy Marison
1
and Ahmad Musir
2
1
Department of Pharmacy, Faculty of Medicine and Health Sciences,State Islamic University, Syarif Hidayatullah, Jakarta
2
Faculty of Pharmacy, University of Pancasila, Jakarta
Keywords: antidiabetes, jengkol rind, male rats, Pithecollobium Jiringa Jack, tolerance glucose.
Abstract: In traditional medicine, the rind of jengkol or skin of jengkol fruit (Pithecellobium jiringa Jack) has been
used by some people to reduce blood glucose levels in some districts of Indonesia. This study aims to
determine the antihyperglycemic effect and glucose tolerance of ethanol extract of the rind of jengkol
(Pithecollobium jiringa Jack) in diabetic rats. Jengkol fruit was bought from Kebonjeruk market, West
Jakarta and determination of jengkol rind was done at the Biology Research Center, Indonesian Institute of
Sciences, Bogor.Indonesia. Jengkol rind was separated from the fruit seeds. Preparation of jengkol rind
extract was done by cold maceration extraction technique using ethanol 70%. The male albino rats that
qualify for the experiment were made into diabetics using the alloxan method. The rats were divided into 7
groups, each group consisted of 5 rats. as positive control for anti-diabetic was used glibenclamide and for
glucose tolerance test was used acarbose, as normal control just given aquadest and for negative control
wass given a solution for suspending the test preparation (1% CMC Na). For extract of jengkol rind was
given low dose ( 24,5 mg/200 gr bw), medium dose (49 mg/200 gr bw) and high dose (196 mg/200 gr bw)
and glucometer tool was used to measure blood sugar levels. Statistical results with ANOVA test and
Kruskal-wallis test showed that small and medium doses of jengkol rind extract had the same antidiabetic
effect and glucose tolerance with positive control and were significantly different to negative controls.
(P≤0.05). and high dose was not significantly different to negative controls (P≥0.05).
1 INTRODUCTION
According to the World Health Organization, In
2017, there are about 150 million people have
diabetes mellitus worldwide, this number may well
double by the year 2025. Majority of this increase
will occur in developing countries and will be due to
population growth, ageing, unhealthy diets, obesity
and sedentary lifestyles. It is estimated in 2025, most
people with diabetes in developed countries will be
aged 65 years or more and in developing countries
most will be in the 45-64 year. Around 1.6 million
people worldwide died due to diabetes in 2017. It is
estimated about 500 million people are living with
diabetes all over the world. By 2045, Therefore, in
recent years, diabetes has become one of the leading
causes of deaths worldwide (WHO, 2017)
There are 2 forms of diabetes that are most
common, i.e. Diabetes (type 1), This is known as
insulin-dependent, in which the pancreas fails to
produce the insulin. Majority of this form develops
in children and adolescents, but is being increasingly
noted later in life. Diabetes (type 2) This is known as
non-insulin-dependent which results from the body's
inability to respond properly to the action of insulin
produced by the pancreas. Type 2 diabetes is around
90% of all diabetes cases worldwide. It occurs most
frequently in adults, there are about 40% of diabetes
sufferers require oral agents for their blood glucose
control, and also there are about 40% need insulin
injections. (WHO, 2017)
Insulin is unaffordable in many poor countries.
On the other hand, the use of synthetic drugs for
diabetes has many side effects. Moreover, oral
diabetes medications rarely work double as a
decrease in blood glucose levels and work as a
glucose tolerance inhibitor (WHO, 2017).
Double work as a decrease in blood glucose
levels and works as a glucose tolerance inhibitor,
only possible on drugs that are sourced from natural
products. Because natural products are usually
chemical compounds that work as diabetes drugs not
Musdja, M., Marison, W. and Musir, A.
Antihyperglycemic Effect and Glucose Tolerance of Ethanol Extract the Rind of Jengkol (Pithecollobium jiringa Jack) in Diabetic Rats.
DOI: 10.5220/0009941322452250
In Proceedings of the 1st International Conference on Recent Innovations (ICRI 2018), pages 2245-2250
ISBN: 978-989-758-458-9
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
2245
only one chemical compound, but can be more than
one chemical compound, namely the form of
synergy of several chemical compounds. Therefore
the discovery of diabetes drugs from natural
products is very necessary (WHO, 2017; Muslim
and Majid, 2010; Zurhana et al., 2018)
Pithecellobium jiringa (Jack) or called as Jengkol
in Indonesia, jering in Malayasia, krakos in
Combodia and niang-yai in Thailand (Muslim N,
2010). The seeds or beans of jengkol fruit is
delicious to make curry or fried with chili. and many
people are addicted to eating jengkol because of its
delicious taste. Generally, the rind of jengkol fruit
seeds or the skin of jengkol fruit is not eat, usually
not used for anything, and just thrown away as
organic waste. (Muslim and Majid, 2010; Zurhana et
al., 2018; Bunawan et al., 2013)
In traditional medicine, usually jengkol used, to
treat toothache, gum pains, chest pains and skin
ailments in the old Indonesia and Malaysian folk.
Raw eaten jengkol fruit seeds are believed to help to
purify the blood and to serve as anti-diabetic agent
and to induce urination. (Bunawan et al., 2013).
As the research was conducted by Ruzilawati et
al (2012) and Zurhana et al (2017), that jengkol fruit
also works as antimicrobial and anti-jamur,
including the bacteria Trychophyton
mentagrophytes, S. aureus, S. epidermidis and M.
gypsum (Zurhana et al., 2018; Ruzilawati et al.,
2012; Charungchitraka et al., 2011)
Jengkol was reported containing chemical
compounds among others : five flavan-3-ol
derivatives which include new flavan-3-ol
gallatesgallocatechin 3‘- and 4‘-O-gallates as well as
gallocatechin 7,3‘- and 7,4‘-di-O-gallates ,
procyanidinds B-3 and B-4 and prodelphinidin B-1,
as well as flavan-3-ols. The metabolites identified
were generally found to be fatty acids, terpenoids,
ally sulphur, vitamin E, Djenkolic acid and alkaloid.
(Bunawan et al., 2013)
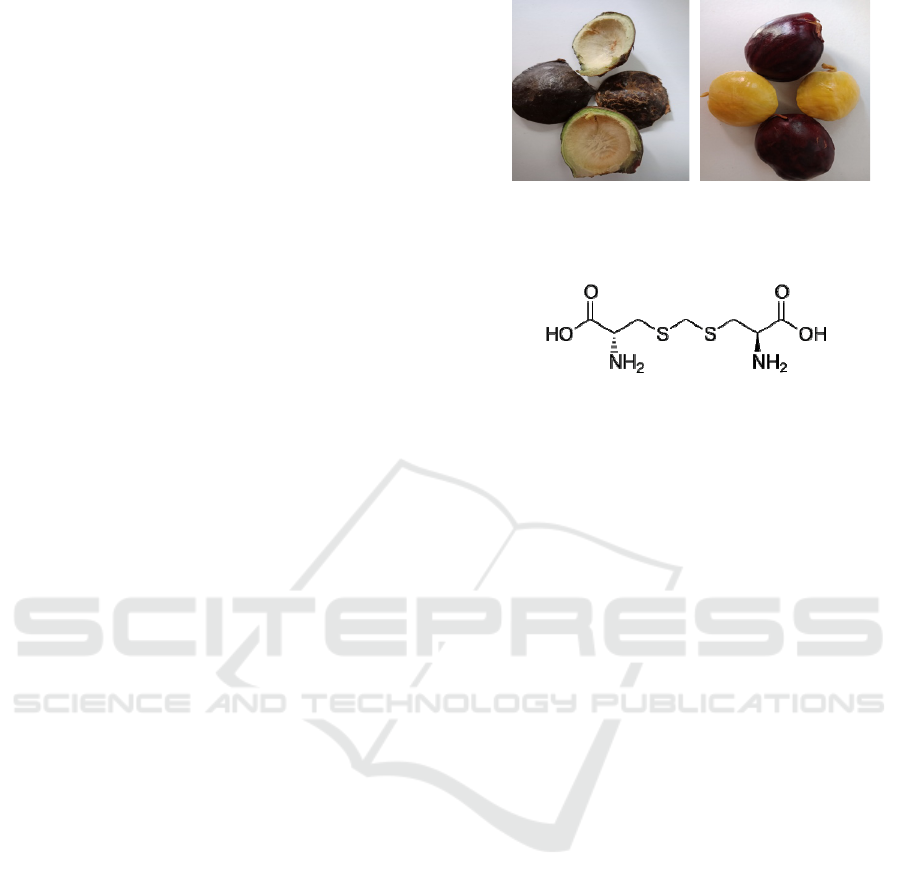
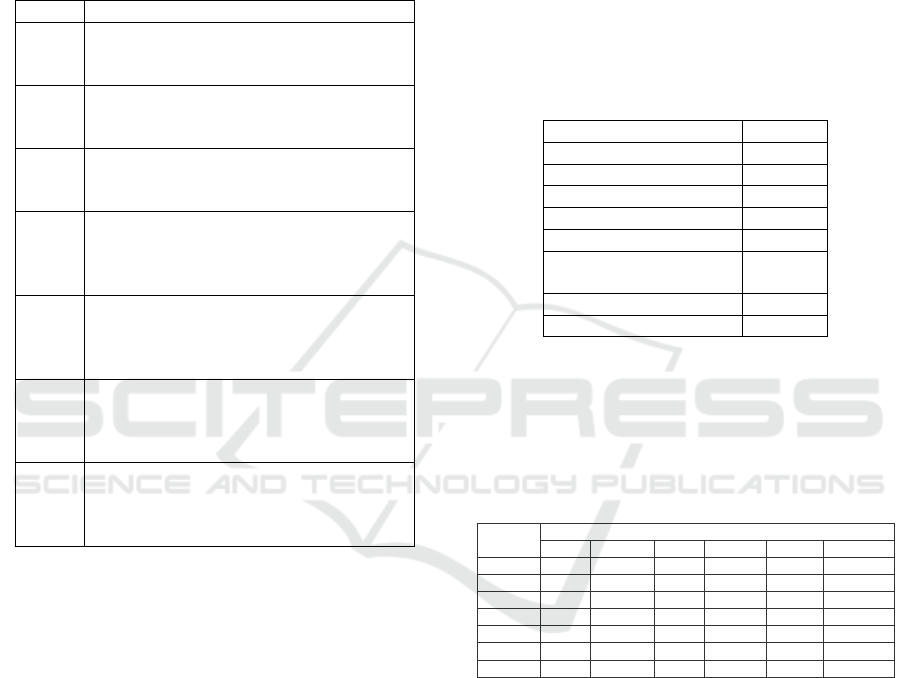
The specific and stinging smell of jengkol is
sourced from djenkolic acid which is contained by
jengkol fruit (figure 2). Because the taste of jengkol
is very delicious, many people consume jengkol
excessively and cause poisoning known as
Djengkolism, In other words, djenkolism is an
uncommon but important cause of acute kidney
injury. It sporadically occurs after an ingestion of the
jengkol bean (Zurhana et al., 2018; Bunawan et al.,
2013).
1a 1b
Figure 1a. the Jengkol rind and 1b. Jengkol seed
Figure 2: Djenkolid Acid
2 METHODS
Jengkol fruit was purchased in the Kebon Jeruk
market, West Jakarta and the taxonomy
determination of plants was carried out at the
Biology Research Center, Indonesian Institute of
Sciences, Bogor, Indonesia.
The making of simplicia was done as follow; A
total of 700 g of jengkol rind powder was extracted
by repeated maceration method by using 70%
ethanol solvent and stirred occasionally until the
solution obtained was clear. The obtained filtrate
was evaporated by using a vacuum evaporator. The
extract obtained was dried in an oven at 70 °C.
Screening of chemical compound groups of
jengkol rind extract were done based on Harbone
methods, in this cases, analysis of chemical
compound groups were done for groups of alkaloid,
flavonoid, saponin, steroid, triterpenoid, tannin,
quinone and essential oil (Harborne, 1998).
The male white rats, strain of Sprague-Dawley
with 3-4 months old (weight 190-250 g) were
acclimatized for two weeks. The rats qualified for
the experiment were divided into 7 groups. each
group consists of 5 rats, before the experiment
begins, the rats was fasted for 10 hours.
The animals were fed with standard pellet diet
and water was given ad libitum. This study was
carried out in the animal house of State Islamic
University, Syarif Hidayatullah Jakarta and this
study was approved by the Institutional Ethical
Committee. The grouping of rats for experiment as
shown in table 1.
ICRI 2018 - International Conference Recent Innovation
2246
The dose of acarbose and glibenclamid given to
rats were calculated based on effective doses for
humans (50-200 mg / kg bw for acarbose and 5 - 10
mg/ kg bw for glibenclamid) and converted based on
the conversion of Paget and Barnes ie the dose for
every 200 g of rat equivalent to 0.018 x human dose
(Watts, 1984)
Table 1: The grouping of rats for experiment
Group Treatment
1 Normal control, given aquadest 3ml/200 g
bw
2 Negative control was made diabetes,
given (50 % glucose, 1%, CMC Na,
aquadest) each 1ml/200 g bw
3 Positive control was made diabetes, given
(acarbose 1,8 mg in1%, CMC Na, 50%
glucose, aquadest) each 1ml/200 g bw
4 Positive control was made diabetes, given
(glibenklamid 0,09 mg in1%, CMC Na,
50% glucose, aquadest) each 1ml/200 g
bw
5 Low dose was made diabetes, given
(jengkol rind extract 24,5 mg in 1% CMC
Na, 50% glukose , aquadest ) each
1ml/200 g bw
6 Medium dose was made diabetes given
given (jengkol rind extract 49 mg in 1%
CMC Na, 50% glukose , aquadest ) each
1ml/200 g bw
7 High dose was made diabetes, given
(jengkol rind extract 98 mg in 1% CMC
Na, 50% glukose , aquadest ) each
1ml/200 g bw
To make rats become diabetic was given alloxan
through intravenous injection. Rat blood
measurements were carried out before giving
alloxan. Alloxan dosage was calculated based on the
effective dose to make the rats become diabetic,
i.e.13 mg / 200 g bw of rat. On the days 7th until
14th usually the blood sugar levels of rats became
stable with diabetes. (Kurniati, 2007). The method
for administering test animals and animal grouping
in more detail is shown in Table 1.
The rats blood were taken through intravenous and
their blood sugar levels were measured by using a
glucometer tool. Furthermore, rat blood was taken at
30, 60, 90, 120, 150 and 180 minutes. Data of blood
glucose level obtained was calculated by using
statistical with methods of Levena, ANOVA and
Kruskal Walis.
3 RESULT AND DISCUSSION
The result of the taxonomic determination of the
plants that was carried out in Biological Research
Center, Indonesian Institute of Sciences showed that
plant was used in this research was Pithecollobium
jiringa Jack
The results of phytochemical screening of methanol
extract of jengkol rind showed that the group of
chemical compounds contained in this plant Was as
shown in Table 2.
Table 2: The content of groups of chemical compounds of
jengkol rind extract
Chemical group Results
Alkaloids +
Flavonoids +
Saponin +
Tannin +
Quinone +
Steroids &
Triterpenoids
+
Essential oil +
Qoumarine -
Results of measurement of blood glucose levels
of test animals before treatment and after treatment
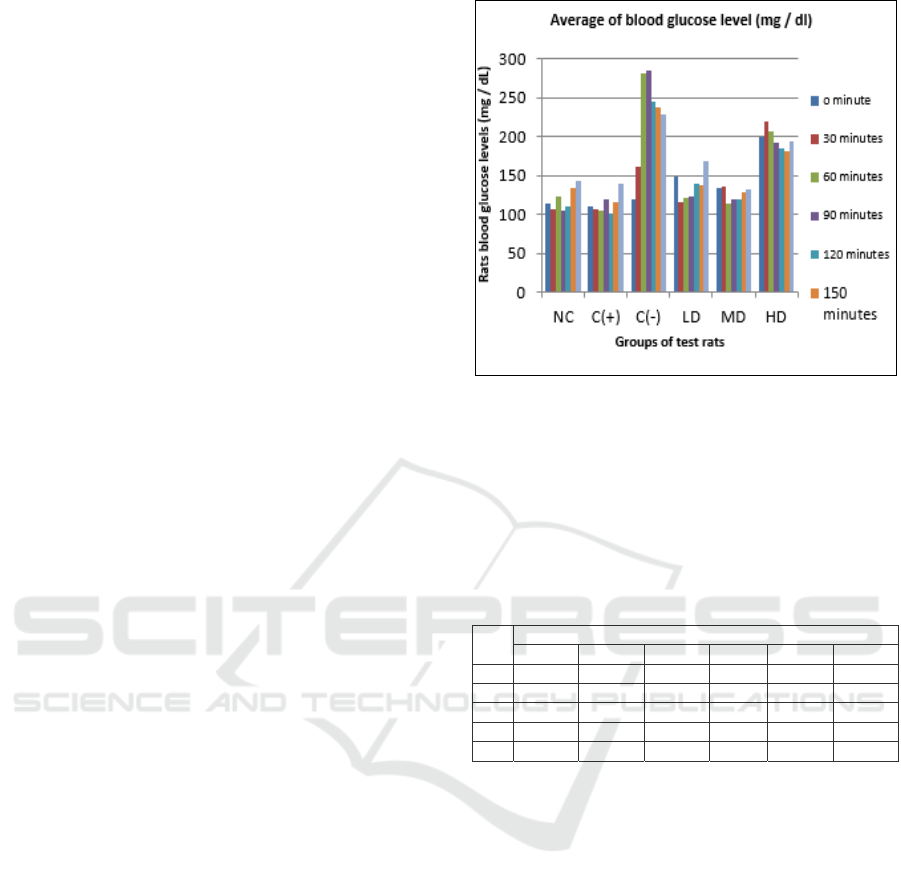
was shown in Table 3 and Figure 1.
Table 3: Results of measurements of average blood
glucose levels for oral glucose tolerance in experimental
rats
minutes
Average of blood glucose level (mg / dl)
NC C(+) C(-) LD MD HD
0 114 110.75 118.5 149 134.5 199.75
30 106.75 107 161.75 115.5 135.5 219.75
60 123 105 280.75 120.75 114.5 206.25
90 105 118.75 285.25 122.25 119.25 192
120 111 100.25 244.5 139.75 119.75 185.25
150 134.25 114.75 237 137.25 128.5 182
180 143.5 139.25 228 168.25 131.75 194
Notes :
NC : Normal Control LD: Low Dose
C(+) : Positive Control MD: Medium Dose
C(-) : Negative Control HD: High Dose
As shown in Table 3 and Figure 1. On the
negative control of rats group, due to glucose
administration in diabetic rats, in observation every
30 minutes, there was a gradual increase in glucose
levels compared to 0 minutes (118.5 mg / dL) with
an increase value for 30, 60, 90 minutes were
161.75; 280.75; 285.25 mg / dL, respectively. Based
on Statistical test was significantly different. Then it
starts to decrease bit by bit at 120, 150, 180 minutes
with a value of 244.5; 237; 228mg / dL,
respectively(P≤0.05).
Antihyperglycemic Effect and Glucose Tolerance of Ethanol Extract the Rind of Jengkol (Pithecollobium jiringa Jack) in Diabetic Rats
2247
In normal group rats, or rats that did not have
diabetes, due to glucose administration there were
no increase in glucose levels. The results of
measurement of glucose levels every 30 minutes to
180 minutes, only fluctuated and based on statistical
tests did not differ significantly (P≥0.05)
In the group of positive control, the rats with
diabetes were given drugs that acted as glucose
tolerance, due to glucose administration in diabetic
rats, no increase in glucose levels. The results of
measurement of glucose levels every 30 minutes to
180 minutes, only fluctuated and based on statistical
tests did not differ significantly (P≥0.05)
On the low dose of jengkol rind extract, the rats
with diabetes, due to administration of jengkol rind
extract, showed decrease in glucose levels compared
to the 0 minute (149 mg / dL) with decrease value
for 30, 60, 90, 120, 150 minutes were 115.5; 120.75;
122.25; 139.75; 137.25 mg / dL, respectively and
increased at 180 minutes with a value of 168.25 mg /
dL.
While, on the middle dose of jengkol rind
extract, the rats with diabetes, showed decrease in
glucose levels compared to the 0 minute (134.5 mg /
dL) with decrease value for 60, 90, 120, 150 and 180
minutes were 114.5; 119.25; 119.75; 128.5; 131.75;
mg / dL, respectively, work effect of this middle
dose was same with work effect of positive control
(acarbose), only different on 180 minutes, where
positive control at 24 days was still high i.e. 139.25
mg/dL or higher than 0 minutes (110.75 mg/dL).
On the high dose of jengkol rind extract, the rats
with diabetes, showed decrease in glucose levels
compared to the 0 minute (199.75mg / dL) with
inrease value for 30, 60, minutes were 219.75;
206.25 mg / dL, respectively and decreased at 90,
120, 150 and 180 minutes with a value of 192;
185.25; 182 and 194mg / dL, respectively. As shown
in Table 3 and Figure 1.
Figure 1. Results of measurements of average blood
glucose levels for oral glucose tolerance in experimental
rats.
Notes :
NC : Normal Control LD: Low Dose
C(+) : Positive Control MD: Medium Dose
C(-) : Negative Control HD: High Dose
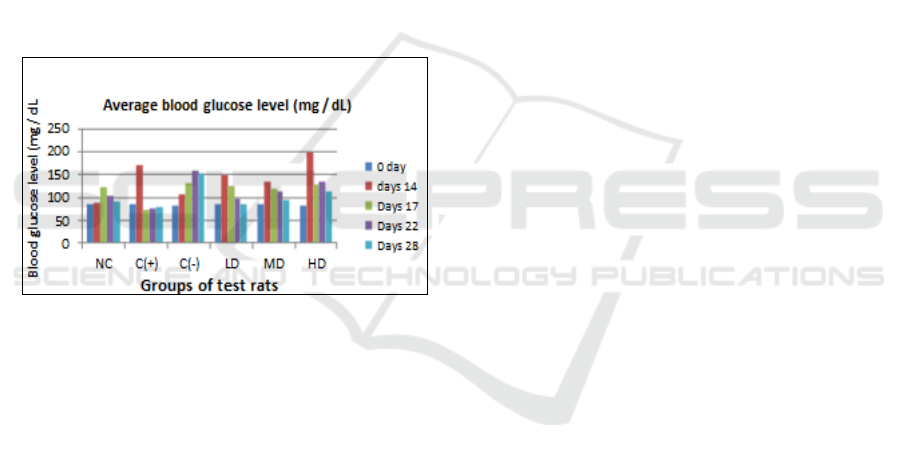
Table 4: Results of measurements of average blood
glucose levels in experimental rats
D
ays Average blood glucose level (mg / dl)
NC C(+) C(-) LD MD HD
0 84.75 85.5 83.75 84.75 86.25 82.75
14 88.5 172.25 106.25 149 134.5 199.75
17 121.5 72.5 130.25 124 117.75 128.75
22 104.25 77 158.25 99 114.5 135.5
28 90.5 78.5 152 84 94.75 111.5
The Results of measurements of average blood
glucose levels in experimental rats, as shown in
Table 4 and Figure 2. On the negative control of rats
group, due to glucose administration in diabetic rats,
on measurement at 14, 17, 22 and 28 days, there
were a gradual increase in glucose levels compared
to 0 day (83.75 mg / dL) with an increase value for
30, 60, 90 minutes were 106.25; 130.25; 158.25 and
152mg / dL, respectively. Based on Statistical test
was significantly different (P≤0.05).
In normal group rats, or rats that did not have
diabetes, due to glucose administration there was no
increase in glucose levels. The results of
measurement of glucose levels at 14, 17, 22 and 28
days, only fluctuated and based on statistical tests
did not differ significantly (P≥0.05)
In the group of positive control, the rats with
diabetes were given glibenclamid that acted to
decrease glucose, due to glibenclamid administration
in diabetic rats, no increase in glucose levels. The
ICRI 2018 - International Conference Recent Innovation
2248
results of measurement of glucose levels at 14, 17,
22 and 28 days, only fluctuated and based on
statistical tests did not differ significantly (P≥0.05)
On the low dose of jengkol rind extract, the rats
with diabetes, due to administration of jengkol rind
extract, showed increase in glucose levels compared
to the 0 day (84.75 mg / dL) with increase value for
14 and 21, days were 149 and 124 mg / dL,
respectively and decreased at 22 and 28 days with
value of 99 and 84 mg / dL. and so for the middle
dose of jengkol rind extract. Work effect of low dose
and middle dose of jengkol rind extract were same
wit work effect of positive control (glibenclamid) in
decrease glucose.
While, on the high dose of jengkol rind extract,
the rats with diabetes, showed glucose levels on days
compared to the 0 day (86.25 mg / dL) with decrease
value for at 14, 17 and 22 days with the value
199.75; 128.75 and 135.5 mg/dL, respectively, then
a little decrease at 22 days, i.e 111.5 mg/dL. As
shown in Table 4 and Figure 2.
Figure 2: Results of measurements of average blood
glucose levels in experimental rats
If this study (Jengkol Rind as an antidibetic) was
compared with the research of Rahanah et al (2011)
by using jengkol seeds as antidiabetic, then jenkol
rind is stronger than jengkol seeds as an antidibetic.
Because jengkol rind extract with a low dose
(24.5 mg / 200 g bw) could reduce blood glucose
levels within 28 days, while in the results of research
of Rahanah et al (2011) Jengkol beans extract could
reduce glucose levels on day 84 or 12 weeks.
Whereas for the effect of glucose tolerance of
jengkol rind extract can also inhibit glucose
tolerance in 48 hours or in 2 days after
administration of jengkol rind extract for moderate
dose or 49 mg / 200 g bw.
On the test for hypoglycemia effect on oral
glucose tolerance ethanol extract of jengkol rind
could reduce blood glucose levels, as shown wit not
significantly differet at low doses of 24.5 mg / 200 g
bw and 49 mg/200 g bw with normal and positive
controls (P≥0.05) and significantly different between
the test dose with negative control (P≤0.05) in the
60
th
and 90th minutes. At high doses it had no effect
because there was no difference significant between
high doses with negative controls in the 60
th
, 90
th
,
120
th
, 150
th
and 180
th
minutes.
On the hypoglycemia effect test, on diabetic rats,
the ethanol extract of jengkol rind was proven to
reduce blood glucose levels, this was shown, with
there was no significantly different at low doses of
24.5 mg / 200 g bw of rats and middle doses of 49
mg / 200 g bw of rats with normal and positive
control (P≥0.05), and there were significantly
different between the test dose with negative control
(P≤0.05) on the 22nd and 28th days. And at high
doses did not have an effect because it was not there
were significantly different between high doses and
negative controls on days 17 and 22.
If this study (Jengkol Rind as an antidibetic) was
compared with the research of Rahanah et al. (2011)
by using jengkol seeds as antidiabetic, then the
jengkol rind is stronger than jengkol seeds as
antidabetic.
Because jengkol extract with a low dose (24.5
mg / 200 g bw) could reduce blood glucose levels
within 28 days, while in the results of research of
Rahanah et al (2011) Jengkol beans extract could
reduce glucose levels on day 84 or 12 weeks.
Whereas for the effect of glucose tolerance, jengkol
rind can also inhibit glucose tolerance in hours or 2
days after administration of jengkol rind extract for
moderate dose (49 mg / 200 g bw). In research of
Rahanah et al., Studied for glucose tolerance testing
were not conducted
Based on research of Yanti et al 2017, Jengkol
protein could reduce interleukin-6 and leptin as
compound for trigger obesity, as we know obesity is
one of trigger diabetes disease. (Yanti et al, 2017)
4 CONCLUSION
Ethanol extract of jengkol (Pithecellobium jiringa
Jack) rind Pram was potential to reduce blood
glucose levels, both with oral glucose tolerance test
and alloxan diabetes test.
REFERENCES
WHO, Global report on diabetes, 2017.
Muslim, N. and Majid, A. (2010). Pithecellobium jiringa:
A traditional medicinal herb. Webmed Central
Complementary Medicine, 1 (12): 1371.
Antihyperglycemic Effect and Glucose Tolerance of Ethanol Extract the Rind of Jengkol (Pithecollobium jiringa Jack) in Diabetic Rats
2249

Zurhana MH, Nurul AO, Aiza H, Shaari D, (2018)
Phytochemical and antimicrobial evaluation of
Pithecellobium jiringa stem barks extracts, Malaysian
Journal of Analytical Sciences, Vol 22 No 1 : 123 –
127. DOI: https://doi.org/10.17576/mjas-2018-2201-
15.
Bunawan, H., Dusik, L., Bunawan, S. N., M. and Amin,
N. (2013). Botany, traditional uses, phytochemistry
and pharmacology of Archidendron jiringa: A
Review. Global Journal of Pharmacology, 7(4): 474-
478.
R
Nahdzatul, S. M., Zeyad, D. N., Abdalrahim, F. A. A.,
Shafaei, A., Norshirin, I., Amin, M. S. A. M. and
Zhari, I. (2012). Antiangiogenesis and antioxidant
activity of ethanol extracts of Pithecellobium jiringa.
BMC Complementary and Alternative Medicine,
12(1): 210.
Ruzilawati, A. B., Imran, A. and Shaida, F. S. (2012).
Effect of Pithecellobium jiringa as antimicrobial
agent. Bangladesh Journal of Pharmacology, 7(2):
131-134.
Charungchitraka, S., Petsoma, A., Sangvanicha, P. and
Karnchanatat, A. (2011). Antifungal and
antibacterial activities of lectin from the seeds of
Archidendron jiringa Nielsen. Food Chemistry, 126
(3): 1025–1032.
Harborne JB. Phytochemical methods: A guide to modern
techniques of plant analysis. 3rd ed. London:
Academic Press; 1998. p. 192-204.
Watts, H.David. 1984.(In bahasa] Terapi medik
(Handbook of Medical Treatment). Edisi ke-17. di
terjemahkan oleh Petrus Lukmanto. Penerbit Buku
Kedokteran EGC, Jakarta; 240 – 241
Yanti, Woenardhy, K., Widjaja, A.Y. and Agustinah,
W.(2017),
Effect of protein fractions from
Pithecellobium jiringa on secretions of interleukin-6
and leptin in 3T3-L1 preadipocytes, /IFRJ 24(5):
2146-2152
ICRI 2018 - International Conference Recent Innovation
2250
