
Adsorption Efficiency of Lead by Sargassum crassifolium in Different
Biomass Size and Dose
Lily Surayya Eka Putri
1
, Eka Syafiqa
1
and Eka Apriliyani
1
1
State Islamic University Syarif Hidayatullah Jakarta, Jl. Ir. H. Djuanda No. 95, Ciputat 15412, Indonesia
Keywords: Adsorption, biomass size and dose, lead, Sargassum crassifolium
Abstract: Lead is the dominant water pollutants emerged in high concentration in a watershed of Jakarta, Indonesia.
Biosorption using macroalgae is the efficient method used to eliminate heavy metals from liquid
waste.Sargassum crassifolium is the original species of macroalgae from Indonesia that was used to analyze
the ability of adsorption of lead (Pb) from industrial wastewater at different biomass size and dose.Samples
collected at Pari and Kotok Besar Island, Seribu Island, North Jakarta, Indonesia were used to adsorb Pb
from industrial wastewater with the biomass size of 10-50 µm and 25-500 µm and biomass dose of 0.1,
0.2,and 0.3 mg/L , using duplicate assessment. The adsorption of S. crassifolium on lead was higher in the
size of 10-50 µm than in 250-500 µm, within 60 minutes oscillation at a particular pH. There was not a
significant differentiation between biomass weight and adsorption capacity (p>0.05). Thus, S. Crassifolium
can be used as an alternative biosorbent which has high efficiency for wastewater treatment, even in less
biomass amount.
1 INTRODUCTION
Many industries whose materials are not widely
used from processes such as discharge as high-color
wastewater, biochemical oxygen demand (BOD),
chemical oxygen demand (COD), pH, temperature,
turbidity and toxic chemicals including heavy metals
(Davies et al , 2003). all of that will be responsible for
the release of heavy metals into the environment
through the production process. the process of direct
and indirect disposal of this waste water into water
bodies pollutes water and affects aquatic organisms.
Industrial wastes usually contain metals and a large
number of potentially harmful compounds. This can
cause environmental pollution and affect ecosystems
(Hussein et al., 2004).
The treatment systems of industrial waste are
many and most of them are physic and chemical
processes. Less biological methods are chosen by
industry to treat the wastewater. One of the
biological methods is through biosorption process
using macroalgae. Various macroalgae are found
throughout the world’s oceans including in
Indonesian water which consists of three basic
colors such as green, brown and red algae
(Muhammad and Nwaedozie, 2011).
The presence of heavy metals in the environment
is a major concern due to the toxicity effect to the
aquatic living organisms (Romera, 2007). Thus,
heavy metals recovery from industrial wastewater is
becoming increasingly important in terms of
neutralizing the hazards of heavy metals.
Many studies on heavy metal adsorption by
macroalgae have largely been restricted to various
species of brown macroalgae (Surjani, 2010; Al
Homaida et al, 2011; Huang and Lin, 2015; Sweetly
et al, 2014), microalgae (Putri et al, 2015),
mushroom (Vimala and Das, 2009), and agriculture
waste (Ashraf et al, 2011). In Indonesia, study of
macroalgae as biosorbent of heavy metal is also
developed which reported adsorption of Cu(II) on
Sargassum crassifolium (Ronaldo et al, 2013),
Cr(III) on Euchema spinosum (Sudiarta and
Diantariani, 2008), Pb in Eucheuma spinosum,
Padina minor and Sargassum crassifolium (Putri,
2016), and Pb(II) on Sargassum duplicatum (Buhani
et al, 2006). However, those studies were
experimented in aqueous solution, and less in
industrial wastewater. Therefore, this study was
done to evaluate the potential of brown algae
Sargassum carssifolium to remove heavy metals
from industrial wastewater. As biosorbent, S.
crassifolium are widely found in Indonesian water
and also has the important advantages including low
Eka Putri, L., Syafiqa, E. and Apriliyani, E.
Adsorption Efficiency of Lead by Sargassum crassifolium in Different Biomass Size and Dose.
DOI: 10.5220/0009935618091814
In Proceedings of the 1st International Conference on Recent Innovations (ICRI 2018), pages 1809-1814
ISBN: 978-989-758-458-9
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1809

cost, the abundance distribution in water, size, high
ability to accumulate metals through its cell wall
(Ashraf et al, 2011).
However, the obstacle of the biosorption
method carried out in previous studies was the
availability of biosorbent raw materials in a
relatively limited in the nature. It is known that to
process 10 L of industrial wastewater it takes 100 g
of dried weight of macroalgae or equal to 1000 g of
wet weight of macroalgae. This means that it takes 1
kg of macroalgae to process 10 L of wastewater.
Meanwhile, the amount of wastewater processed by
an industry reaches thousands of liters, so a lot of
macroalgae is needed. Accommodating this
problem, the size and dose of biomass will be an
important aspect. This study focused on the size and
dose of S. crassifolium and analyzing the adsorption
capacity on lead form industrial wastewater. A small
amounts of biomass used will result in cutting costs
for proper industrial wastewater treatment. In
addition, it also conserves macroalgae in the nature
and provides an alternative increase for the economy
of coastal communities.
2 MATERIALS AND METHOD
In this research, it will be conducted at the integrated
laboratory center of the UIN faculty of science and
technology, which will be held in May until October
2018. The macroalgae samples used in this study are
Sargassum crassifolium which is transported from
water around Pari Island and Big Kotok, Seribu
Kepulauan, North Jakarta, Indonesia to the
laboratory. Industrial wastewater is provided from
hazardous waste processing companies located in
Cileungsi, Bogor.
2.1 Preparation of Biosorbent
Before being taken to the laboratory, all samples will
be put into a zip plastic bag, then washed with
seawater to clean from sediments or small organisms
trapped in the macroalgae. henceforth in the
laboratory, all samples were washed and rinsed
again using distilled water then dried in an oven at
50 C for 24 hours to get a stable weight. Dry
samples were grounded with mortars and filtered in
sizes 250-500 μm and biosorbents were ready for
use. Another 10-50 μm size was prepared at
BATAN (National Agency for Nuclear Energy in
Indonesia) using a milling device with 12 balls for 1
hour process.
2.2 Adsorption Test
The experimental design used was Completely
Randomized Design with two (2) replications. Three
variables were tested in this study, including pH,
size and weight of biomass. The variety of biomass
size was 250-500 µm (sa) and 10-50 µm (sb)
whether for biomass dose was 0.1, 0.2, and 0.3 ppm
were examined. All samples were tested in pH 7 and
9 and were oscillated for 60 minutes (Putri, 2016).
The correlation between size and weight of biomass
with adsorption efficiency was analyzed using one-
way ANOVA.
Firstly, for the size 250-500 µm, 0.1, 0.2 and 0.3
g of the dried S. crassifolium was put into 50 ml
wastewater respectively and stirred for 60 minutes
using a magnetic stirrer at 200 rpm. The solution pH
was adjusted to 7 and 9 with NaOH (0.1 M). Then,
the solution were filtered with Whatman paper BF/C
code and the filtrate was analyzed using ICP to
determine the metals concentration at 543 nm
wavelength. The experiment was repeated in the size
10-50 µm.
Metals uptake and removal were calculated as
the difference in the metal concentration(s) before
and after sorption (Ok et al, 2007), according to Eq.
(1).
q = ( ) x
(1)
R
=
x 100% (2)
Where q = metal adsorption (mg/g); M = dry
biomass (g); V = volume of the initial lead solution
(L); C
i
= initial concentration of lead in aquatic
solution (mg/L); C
f
= final concentration of lead in
the aquatic solution (mg/L) at given time (t; min); R
= removal percentage (%).
3 RESULTS AND DISCUSSION
3.1 Lead Adsorption
The original strain of Indonesian marine brown
algae Sargassum crassifolium was examined for lead
removal at different variation of size of biomass,
250-500 µm (sa) and 10-50 µm (sb), and also
different biomass dose, namely 0.1, 0.2, and 0.3 g.
Different pH was also tested which were pH 7 and 9.
Most of studies were examined adsorption of lead in
aqueous solution (Putri, 2016; Sweetly et al, 2014;
ICRI 2018 - International Conference Recent Innovation
1810
Vimala and Das, 2009) using macroalgae and also
mushrooms.
The concentration of hydrogen ions is one of the
important parameters in adsorption process which
affects the ionization degree of absorbate during the
reaction and replacement of positive ion in active
sites of cell wall (Sudiarta and Diantariani, 2008).
Figure 1 showed that S. crassifolium in aqueos
solution could remove 98.4% of lead at 60 minutes
oscillation. This rapid metal adsorption for
Sargassum was due to the active binding sites
owned by macroalgae such as –COOH, –OH and –
NH
2
(Wang and Chen, 2009). This contact time was
further used for the study in wastewater solution.
99
98.5
Removal
%
98
97
97.5
96.5
96
95.5
10 20 30 45 60 90 120
time (minute)
Figure 1: Effect of contact time on the adsorption
of lead (Putri, 2016)
Similar findings for adsorption of heavy metals
such as chromium, nickel and lead by other
adsorbents have been reported by Das and Mondal,
2011; Singh and Singh, 2012 who have shown a
balance with achieving 60 minutes. The presence of
a fast adsorption rate is likely due to the increasing
number of empty sites available for adsorption and
is usually controlled by the diffusion process from
bulk to surface (Agarry and Ogunleye, 2014).
Adsorption after 60 minutes may be a process that is
controlled by attachments because the adsorption
site is less available in the macroalase cell wall
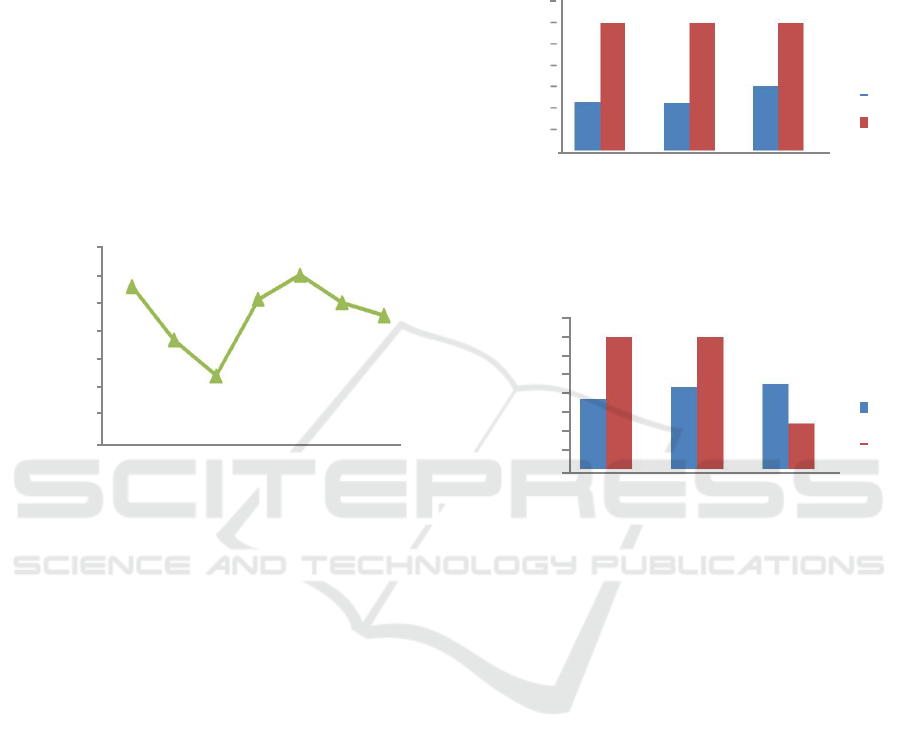
Figure 2 and 3 shows the observed removal
percentage for the assayed Pb ion in the wastewater
solution by S. crassifolium at various size and
weight of biomass and at pH 7 and pH 9. The
percentage removal or adsorption efficiency of Pb
from the wastewater by dried S. crassifolium
biomass was found to be 98.11-98.5% (sa) and
99.99% (sb) in various dose of biomass at pH 7, as
shown in Fig. 2. At pH 9, the removal percentage
almost the same as at pH 7 which was 98.34-98.76
(sa) and 97.71-99.99% (sb) in various biomass dose.
This was evidenced by statistical tests that there was
no difference in the ability of lead adsorption on
both biomass size (p>0.05) and also at various dose
of biomass (p>0.05).
100.5
100
99.5
R
99
%
98.5
sa
98
sb
97.5
97
0.1 0.2 0.3
weight of biomass (g)
Figure 2: Adsorption capacity on Pb at pH 7
100.5
100
99.5
R
99
98.5
%
sa
98
97. 5
sb
97
96.5
0.1 0.2 0.3
weight of biomass (g)
Figure 3: Adsorption capacity on Pb at pH 9
This result was different from the study
conducted by Lee and Park (2012) which reported
pH 4 was the maximum adsorption of lead in
aqueous solution. Similar result was also reported
by Vieira et al (2007), Nessim et al (2011), Sweetly
et al (2014) and Putri (2016) which was pH 4.5, pH
4-5.5 and pH 5 respectively, showed the optimum
lead removal.
Those studies were in aqueous solution which is
the heavy metals were adjusted in specific
concentration to be tested the capability of
adsorption. On contrary to wastewater solution
which is used directly without adjustment of the
concentration. The heavy metals contained in the
wastewater were the mixture of various types of
wastes from the various industries. Therefore, it can
be presumed that the pH condition was seemly
different in aqueous solution and wastewater. There
are a lot of types of heavy metals mixed together in a
very high concentration and this causes the pH
increases. The pH lower than 3.0, the removal of
lead was inhibited possibly as result of a competition
Adsorption Efficiency of Lead by Sargassum crassifolium in Different Biomass Size and Dose
1811

between lead and hydrogen ions and when the pH
increased, the negative charge density on the
macroalgae will also increased due to the
deprotonation of the metal binding sites, which
resulted in increasing the lead adsorption (Vieira et
al, 2007; Nessim et al, 2011).
The most important parameters of biosorption is
the pH (Dubey el, 2014) and regarding to Sargassum
sp. which contains many ionizable groups (carboxyl
groups), it is liable to be influenced by the pH of the
medium which will play a role in binding metallic
ions (Sweetly et al, 2014). The higher the pH value,
the higher the dissociation if the free sites for the
binding process can be produced, and this occurred
at pH values over 5.5 (Vieira et al, 2007). That is
supported by Ho (2005) which reported pH values in
the range of 4.0 to 7.0 were adequate for lead
binding. These values are similar to those obtained
in the present research, of which was pH 7 the
predominance of lead precipitation.
The biomass size, 250-500 µm and 10-50 µm
tested in the present study, showed no significant
differentiation in adsorption efficiency, nevertheless
achieved maximum removal of lead at size 10-50
µm which was higher than at size 250-500 µm. This
is supported by the study conducted by Cossich et al
(2002) which found the biosorbent size did not
influence the capacity and rate of lead removal.
However, this is very important for the industry
because it is related to the cost of processing waste.
Meanwhile, the biomass dose used in the
present study was 0.1, 0.2 and 0.3 g, was not also
showed significant different with removal efficiency
of lead in wastewater solution. This present study
showed that the removal efficiency of lead from
wastewater was not improved with the increasing
dose of assayed biomass. This result was contrary to
Dubey et al (2014) reported that the removal
efficiency was improved with the increasing dose of
biomass 0.25-1.5 g as more surface area in cell wall
of biomass was available for adsorption due to
enhance in the active sites. Leusch et al. (1995) also
found that larger biomass particles of Sargassum
fluitans and Ascophylum nodosum had higher metal
uptake than smaller particles in the case of cadmium,
copper, nickel, lead and zinc. However, these can be
explained by the study by Jia et al (2003) that was
the smaller particles provide a larger surface area for
the attachment of chemical reaction and a shorter
diffusional path for the substrates which supported
the findings of the present study. However, the
findings of this study were in accordance with
observation made by Sweetly et al (2014) in the
uptake of lead by S. myriocystum which decreased
gradually with increased concentration greater than
2g. Vieira et al (2007) was also found the same
results as our findings. Thus, the influence of
biosorbent size on metal adsorption apparently is a
function of both the type of biomass and the metal
ion.
The initial concentration on lead was 13.09
mg/L and after treated by S. crassifolium the final
concentration was ranged 0.001-9.52 mg/L. The
capability of S. crassifolium was definitely high.
Despite, the final concentration of lead at 0.1 and 0.2
dose and size 10-50 µm was below the permissive
level (0.03 mg/L) which is set in the Government
Regulation of Republic of Indonesia No. 82/2001 on
Water Quality Treatment and Water Pollution
Control. The high adsorption ability and achieving
safe concentration to be discharged into the waters
are the advantages of using macrolgae for industrial
wastewater treatment and also environmentally
friendly ways.
3.2 Future Trends
A natural environmentally friendly biopolymer
recently is definitely found its place among the most
important carbohydrates. It is now used effectively
in a wastewater treatment. One of them is guar gum
silica nano-composites were effectively used in the
removal of Cd (II) from aqueous solution at pH 8,
contact time 2 hours, temperature 30
o
C and
adsorbent dose 10 mg (Singh and Singh, 2015).
Other novel study was hydrogel based on guar gum
(Abdel-Halim and Al Deyab, 2011; Khan et al,
2017) economic and ecofriendly biocomposite for
removal of hexavalent chromium ion Cr (VI).
Utilization of nanocomposite based on
biopolymers is the future developed biosorbent with
the environmental and economic considerations on
replacing the synthetic polymers. Nanocomposite of
biomass is also developed well such as Zhao et al
(2011) for chitosan. Our future research targets are
to prepare nanocomposite from macroalgae and
developed hydrogel based on macroalgae for
wastewater treatment, particularly for heavy metals
uptake. Therefore, environmental quality could be
prevented earlier and the nanocomposite
biotechnology can help industries for waste
treatment process efficiently.
ICRI 2018 - International Conference Recent Innovation
1812

4 CONCLUSION
The biological method for wastewater treatment has
been used as the efficiency on cost and time to
adsorb heavy metals, which in addition showed high
adsorption capability on heavy metal. The original
species of Indonesian marine macroalgae Indonesia
Sargassum crassifolium exhibited high lead uptake
from industrial wastewater reached almost 99.99%
in smaller size and dose of biomass at pH 7 and 9 for
60 minutes oscillation. Size and dose of biomass was
mentioned and the different pH was analyzed. It was
established that smaller size and dose of biomass
could alleviate the adsorption capacity of heavy
metals in industrial wastewater. This indicated that
S. crassifolium belonging to Phaeophyta division
could be chosen as an alternative environmentally-
friendly method for wastewater which mostly using
chemical methods.
REFERENCES
Abdel-Halem, E.S. & Al-Deyab, S.S., 2011. Hydrogel
from crosslinked polyacrilamide/guar gum graft
copolymer for sorption hexavalent chromium ion.
Carbohy Poly, 86, pp. 1306-1312.
Agarry, S.E and Ogunleye, O., 2014. Chemically treated
kolanut pod as low cost natural adsorbent for the
removal of 2, 4 dinitrophenol from synthetic wastewater:
batch equilibrium, kinetic and thermodynamic modelling
studies. Turkish Journal of Engineering and
Environmental Science, 38, pp. 11-40.
Al-Homaidan, A.A., Al-Ghanayem, A.A. and Al-Khalifa,
A.H., 2011. Green algae as bioindicator of heavy metal
pollution in Wadi Hanifah Stream, Riyadh, Saudi
Arabia. Int. J. Water Resour. Arid Environ., 1(1), pp. 10-
15.
Ashraf, M.A., Mahmood, K. and Wajid, A., 2011. Study
of low cost for biosorption of heavy metals. In
Proceeding of International Conference on Food
Engineering and Biotechnology IPCBEE 9, Singapore,
pp. 60-68.
Buhani, Suharso and Sembiring, Z., 2006. Biosorption of
Pb (II), Cu (II) and Cd (II) ions on Sargassum
duplicatum immobilized silica gel matrix. Indo. J.
Chem., 6 (3), pp. 245-250.
Cossich, E.S., Tavares, C.R.G., and Ravagnani, T.M.K.,
2002. Biosorption of chromium(III) by Sargassum sp.
biomass. EJB Electronic Journal of Biotechnology,
Vol.5, No. 2, pp. 133-140.
Das, B. and Mondal, N.K., 2011. Calcareous Soil as a
New Adsorbent to Remove Lead from Aqueous
Solution: Equilibrium, Kinetic and Thermodynamic
Study. Universal Journal of Environmental Research
and Technology, 1(4), pp. 515-530.
Davis, T.A., Volesky, B. and Mucci, A., 2003. A review
of the biochemistry of heavy metal biosorption by brown
algae. Water Res., 37, pp. 4311-4330.
Dubey, A., Mishra, A., and Singhal, S., 2014. Application
of dried plant biomass as novel low-cost adsorbent for
removal of cadmium from aqueous solution. Int. J.
Environ. Sci. Technol., 11, pp.1043–1050.
Huang, S. and Lin, G., 2015. Biosorption of Hg(II) and
Cu(II) by biomass of dried Sargassum fusiforme in
aquatic Solution. J. Environ. Health Sci. Eng., 13 (21),
pp. 1-8.
Hussein H., Ibrahim, S.F., Kandeel, K. and Moawad, H.
(2004). Biosorption of Heavy Metals from Wastewater
using Pseudomonas sp. Electronic Journal of
Biotechnology, 7(1), pp.38-46.
Indonesian Government Regulation No. 82 year 2001, The
Treatment of Water Quality and Water Pollution
Control.
Jia, H., Zhu, G. and Wang, P., 2003. Catalytic behaviors
of enzymes attached to nanoparticles; the effect of
particle mobility. Biotechnol. Bioeng., 84, pp. 406-414.
Khan, T.A., Nazir, M., Ali, I., and Kumar, A., 2017.
Removal of chromium (VI) from aqueous solution using
guar gum-nano zinc oxide biocomposite adsorbent. Arab
J. Chem., Vol 10, Suppl. 2, pp. 2388-2398.
Lee, S.H. and Park. C.H., 2012.
Biosorption of heavy
metal ions by brown seaweeds from Southern Coast of
Korea. Biotech. and Bioprocess Eng., Vol. 17, Issue. 4,
pp. 853-861.
Leusch,
A., Holah, Z.R. and Volesky, B., 1995.
Biosorption of heavy metals (Cd, Cu, Ni, Pb, Zn) by
chemically-reinforced biomass of marine algae. Journal
of Chemical and Technology Biotechnology, Vol. 62, pp.
279-288.
Muhammad,
M.N.I. and Nwaedozie, J.M., 2011.
Application of Marine Biomass for the Removal of
Metals from Industrial Wastewater. Proceedings of the
First International Technology, Education and
Environment Conference African Society for Scientific
Research (ASSR).
Nessim, R.B., Bassiouny, A.R., Zaki, H.R., Moawad,
M.N. & Kandeel, K.M., 2011. Biosorption of lead and
cadmium using marine algae. Chemistry and Ecology,
27(6), pp. 579-594.
Ok, Y.S., Yang, J.E., Zhang, Y.S., Kim, S.J. and Chung,
D.Y., 2007. Heavy metals adsorption by a formulated
zeolit-portland cement mixture. J. Hazard. Mater., 147,
pp. 91-96.
Putri, L.S.E., 2016. Biosorption of Lead Using
Macroalgae Eucheuma spinosum, Padina minor and
Sargassum crassifolium in Aqueous Solution. Asian
Journal of Applied Sciences, Volume 4, Issue 2, pp.
520-525.
Putri, L.S.E., Fauziah and Dasumiati, 2015. Biosorption
ability of microalgae Scenedesmus dimorphus for Cr
(VI) and Cd in aqueous solution. Adv. Sci. Lett., 21, pp.
196-198.
Romera, R., Gonzales, F., Ballester, A., Blazquez , M.L.
and Munoz, J.A., 2006. Biosorption with algae: A
statistical review. Crit. Rev. Biotechnol., 26, pp. 223-
235.
Adsorption Efficiency of Lead by Sargassum crassifolium in Different Biomass Size and Dose
1813

Ronaldo, I.H., Silalahi, N, Wahyuni, 2013. Adsorption of
Cu (II) ion using brown algae biomass encapsulated
aqua-silica gel. JKK, 2 (3), pp. 148-152.
Singh, S.R. and Singh, A.P., 2012. Adsorption of heavy
metals from waste waters on tea waste. Global Journal
of Research Engineering, 12 (1), pp. 19-22.
Singh, V. and Singh, S.K., 2015. Cadmium (II) removal
from aqueous solution using guar gum-silica
nanocomposite. Adv. Mater. Lett., 6, pp. 607-615.
Sudiarta, I.W. and Diantariani, N.P., 20008. Biosorption
of Cr (III) on biosorbentof seaweed Euchema spinosum.
Indo. J. Chem., 8 (1), pp. 78-82.
Surjani, B.A., 2010. Heavy Metals removal from
Industries Wastewater by using Seaweed through
Biosorption Process. [Unpublished Thesis]: Faculty of
Civil Engineering and Earth Resources, University
Malaysia Pahang.
Sweetly, D.J., Sangeetha, K. and Suganthi, B., 2014.
Biosorption of heavy metal lead from aqueous solution
by non-living biomass Sargassum myriocystum.
IJAIEM., 3 (4), pp. 39-45.
Vieira, D.M., Costa, A.C.A.D., Henriques, C.A., 2007.
Biosorption of lead by the brown seaweed Sargassum
filipendula – batch and continuous pilot studies. Electric
journal of Biotechnology, 10(3), pp. 368-375.
Vimala, R. and Das, N., 2009. Biosorption cadmium (II)
and lead (II) from aqueous solutions using mushrooms:
A comparative study. J. Hazard. Mater., 168, pp. 376-
382.
Wang, J. and Chen, C., 2009. Biosorbents for heavy
metals removal and their future. Biotechnol. Adv., 27,
pp. 195-226.
Zhao, L.M., Shi, L.U., Zhang, Z.L., Chen, J.M., Shi, D.D.,
Yang, J. and Tang, Z.X., 2011. Preparation and
application of chitosan nanoparticles and nanofibers.
Brazilian Journal of Chemical Engineering, Vol. 28, No.
3, pp. 353 – 362.
ICRI 2018 - International Conference Recent Innovation
1814
