
Technology of Iron Hardening by using the Pressure and Heating
Technique
Sutrisno
1
, Agus Budiono
1
and Tati Zera
1
1
Program Studi Fisika ,Fakultas Sains dan Teknologi, UIN Syarif Hidayatullah Jakarta, Banten, Indonesia
Keywords: technology; surface hardening; iron
Abstract: Iron surface hardening technology is done with a particular technique to minimize oxygen in order to avoid
the formation of oxide compounds that can inhibit the diffusion process and reduce the reactivity between
the iron and the reinforcement material. Conventionally, this technique is done by creating an inert gas
conditions using argon gas flow, or it can also be done by creating a system under vacuum conditions during
heating. This technique is operationally costly because it must provide argon gas or vacuum machines are
expensive. This study is intended to reduce the magnitude of these costs using the new methods with less
operational costs. In this study, iron surface hardening process conducted by using mechanical pressure and
heating without vacuum or argon gas flow. Samples iron S45C to be hardened is placed in a container
shaped cylindrical tube made of 304 stainless steel and covered with a powder mixture consisting of 5%
boron carbide (B
4
C), 90% of silicon carbide (SiC), and 5% potassium boron fluoride (KBF
4
) under certain
pressure. Samples were prepared is heated at a temperature of 700, 800, and 900
o
C with heating time for 8
hours. Layer formed on the metal surface can be identified as a layer of iron borides I (FeB) on the surface
and a layer of iron borides II (Fe
2
B) at certain depths. From the micro hardness test results using Vikers
identor expected to obtain micro hardness value is greater than the base sample so it can be used as a raw
material component machining.
1 INTRODUCTION
The results of research published by the German
Federal Ministry of Research and Technology
reported that the loss was economically due to
abrasion and material wear of more than 10 billion
DM and this figure is close to 1% of the state's
budget (Walter, 1981
). Every year large economic
losses are also experienced by some industries
caused by corrosion and wear damage to machinery
and its components. The condition triggers the
demand for improved surface performance of
metallic materials whose impact has brought about
advancements in the field of surface development
technologies
(Suwattananon, 2005).
The role of the surface of metal material is very
important because it is the part that directly in
contact with the environment. To maintain this role,
efforts should be made to improve the surface
resistance to environmental influences has a longer
lifetime. The damaging effects of the environment
on the surface of metallic materials include wear,
corrosion, oxidation, and collision (
Sugondo, 2010).
In addition to counteracting destructive
environmental influences, the role of metal surfaces
can also be increased so as to have added value and
their usefulness can be extended to other areas.
Carbon steel metal material has been widely
used in various components in the fields of industry,
agriculture, machinery, and automotive (Bintang,
2005). The metal has advantages and disadvantages.
Based on carbon composition, carbon steel can be
grouped into several parts, including low carbon
steel with carbon composition of 0.08 to 0.35%,
medium carbon steel with carbon composition 0.35
to 0.50%, and high carbon steel with composition
carbon 09.55 to 1.70% (Smallman, 1999).
Low carbon steel has several advantages,
including strong and ductile, easy to do with
machining, easy to shape, easy to weld, cheap price,
and many available in the market. In addition, low
carbon steels also have disadvantages such as
relatively low hardness, low wear resistance, no
resistance to corrosion attacks, and easilyoxidized so
that pearlite phase decomposition occurs at high
temperatures
(Bintang, 2005).
Sutrisno, ., Budiono, A. and Zera, T.
Technology of Iron Hardening by using the Pressure and Heating Technique.
DOI: 10.5220/0009928729452951
In Proceedings of the 1st International Conference on Recent Innovations (ICRI 2018), pages 2945-2951
ISBN: 978-989-758-458-9
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
2945

The surface hardening can be performed on low
carbon steels so that the material obtained has a
higher hardness when compared to the hardness of
the original material but remains strong and ductile.
In the process of increasing the hardness occurs
phase changes on the surface of low carbon steel,
whereas in the basic material structure does not
occur changing the phase (Bintang, 2005) .
There are many methods that can be used to
improve the surface performance of metallic
materials as a result of surface development
technologies. In general the method can be done in
two ways. First, the method does not alter the
chemical composition of a base material called
thermal heat treatment, such as the flame hardening
and induction hardening methods. Second, the way
is done by changing the chemical composition of the
base material called thermochemical heat treatment,
for example carburization method, nitriding,
carbonitriding, and boronizing (Bintang, 2005). This
second way is mostly done in the industrial world at
a certain temperature so known as thermochemical
treatment (Roumiana, 2008).
Among the four methods mentioned
above,boronization is the most superior method
because it can provide better results on the surface
performance of metallic materials (Anil Kumar
Sinha, 1990). Boronization is a thermochemical
process in surface hardening that can be applied to a
variety of metal materials, both ferrous and non-
ferrous metals. Boronization methods on the surface
of metallic materials are generally carried out at
temperatures of 700
o
C up to 1000
o
C for 1 to 12
hours and can be carried out in solid, liquid, and gas
media (Anil Kumar Sinha, 1990).
As a result of the process of boronization on low
carbon steel will form a layer of boride iron with the
possibility of a single phase FeB, Fe
2
B, or FeB and
Fe
2
B combined phases (Gopalakrisnan, 2001). The
formation of single phase both FeB and Fe
2
B is
more desirable because it will produce better
mechanical properties than the combined phases. In
addition, other constituent elements in low carbon
steel alloys also have the possibility to form a boride
phase so the other phases (Setiawan, 2010) will
occur.
The method of boronization is done by
minimizing oxygen technique to avoid the formation
of oxide compounds that can inhibit diffusion
process and reduce the reactivity between iron and
boron (Martini, 2004). The technique is usually
performed by creating an inert gas condition with an
argon gas flow, or it can also be done by making a
vacuum during heat treatment (Martini, 2004). In
addition it can also be carried out under atmospheric
pressure conditions during heating.
Roumiana et al in 2008 has carried out
boronization of powder on low carbon steel AISI
1018 with a mixture of B
4
C and KBF
4
powders. The
heating process was carried out at 850° C for 4 hours
under argon gas conditions and resulted FeB and
Fe
2
B borate layers with 75 to 80 μmthickness and
2250 HK hardness. The same study has also been
done by Sugondo in 2007 on St37 steel resulted
FeB and Fe
2
B borate layers with hardness reaching
1400 HV (Sugondo, 2010).
Boronization methods under vacuum have been
performed by Martini et al in 2004 at 99.9% pure
iron with different powders B
4
C, SiC, and KBF
4
compositions. The sample heating was carried out at
850
o
C for 15 hoursformedFeB and Fe
2
B using 3
different composition types for B
4
C powder 10%,
100%, and 90% weight (Martini, 2004). Then in
2006 with the same technique Dybkov et al do
boronization on iron alloy 25% Cr resulted FeB and
Fe2B borate layers using mixed powders B
4
C and
KBF
4
. The micro hardness that occurs in the boride
layer is 18 Gpa (Dyvkov, 2006). Both inert gas and
vacuum engineering need the high cost and difficult
to do in a business-oriented industry because of its
less practical use.
To overcome these conditions need to find a
solution so that the heating technique can be done
simply and the implementation is more practical
without reducing the quality of the expected
results.In this research will be applied the pressure
and heating technique with a certain pressure on
boronization powder during the heating process
without reducing the quality of expected results. As
the basic material selected S45C low carbon steel
which is cheap and easily available in the market.
Neither the first method involves altering the
chemical composition of the base material nor the
second way by changing the chemical composition
of the base material, all by a vacuum or by an inert
gas stream. If the equipment is not good then
leakage will often occur so that the hardening
process on the sample that is scientifically
manifested in the form / phenomenon of diffusion
can not take place. Both inert gas engineering and
vacuum engineering both cost considerable and
difficult to do in a business-oriented industry
because of its less practical use.
2 RESEARCH METHOD
The basic materials used as the basic samples are
iron S45C. Iron S45C consists of elements 0.42 -
ICRI 2018 - International Conference Recent Innovation
2946

0.48 wt% C, 0.15 - 0.45 wt% Si, 0.60 to 0.90 Mn,
0.03 P, and 0.035 S (Setiawan, 2010). To know the
mechanicalproperties of the sample before
boronizing, sample characterized by XRF, XRD,
and micro hardness testing. The surface of the
sample to be diboronized first cleaned from the
impurity layer and smoothed in order to obtain
maximum results.
Figure 1. Basic sample of soft iron type S45C
The composition of the powder mixture used
includes 50wt% B
4
C, 5wt% KBF
4
, and 45wt% SiC.
The mixed powder is inserted into the iron base
sample S45C, and pressed with a pressure of 15 tons
and then heated in a furnace with a temperature of
700, 800, and 900
o
C for 8 hour heating time.A
container containing a working sample that has been
sealed with a boron powder is fed into the furnace to
be heated. Figure 2 shows the container that has
contained the working sample put in the furnace and
is ready to be heated.
a
b
c
Technology of Iron Hardening by using the Pressure and Heating Technique
2947

d
Figure 2. The sample into cylinder and lead (a), boriding
powder covered the sample (b), cylindrical container
claimed by pressure (c) container in the furnace is heated.
By applying pressure to the boronization
powder, the heating of the container in the furnace is
carried out directly without using a vacuum system
or draining the argon gas into the furnace.The
heating setting begins by raising the heating
temperature from room temperature to 700°C within
30 minutes. After reaching the temperature 700°C
held for 8 hours. This process is carried out also for
temperature of 800 and 900
o
C during 8 hours . When
finished the heater is stopped and the cooling is done
naturally at room temperature.
If it has been shown room temperature, the
boronized treated work sample is removed from the
heater.To find out the changes, the already
boronized sample is characterized and tested.
Characterization was carried out to find out the
change of micro structure, whereas the test was
performed to see micro hardness at the cross section
of the boride layer. The diffraction pattern of the
obtained XRD data was analyzed using GSAS to
determine the phase composition of the boride layer.
In a sample that has been boronized observations
of microstructure to see the morphology of the
boride layer formed. The observations use a Nikon
optical microscope with 200 times magnification.
The depth of the boronized borate layer is obtained
from the average depth of sawtooth (Setiawan,
2010). All of the boronized samples were tested for
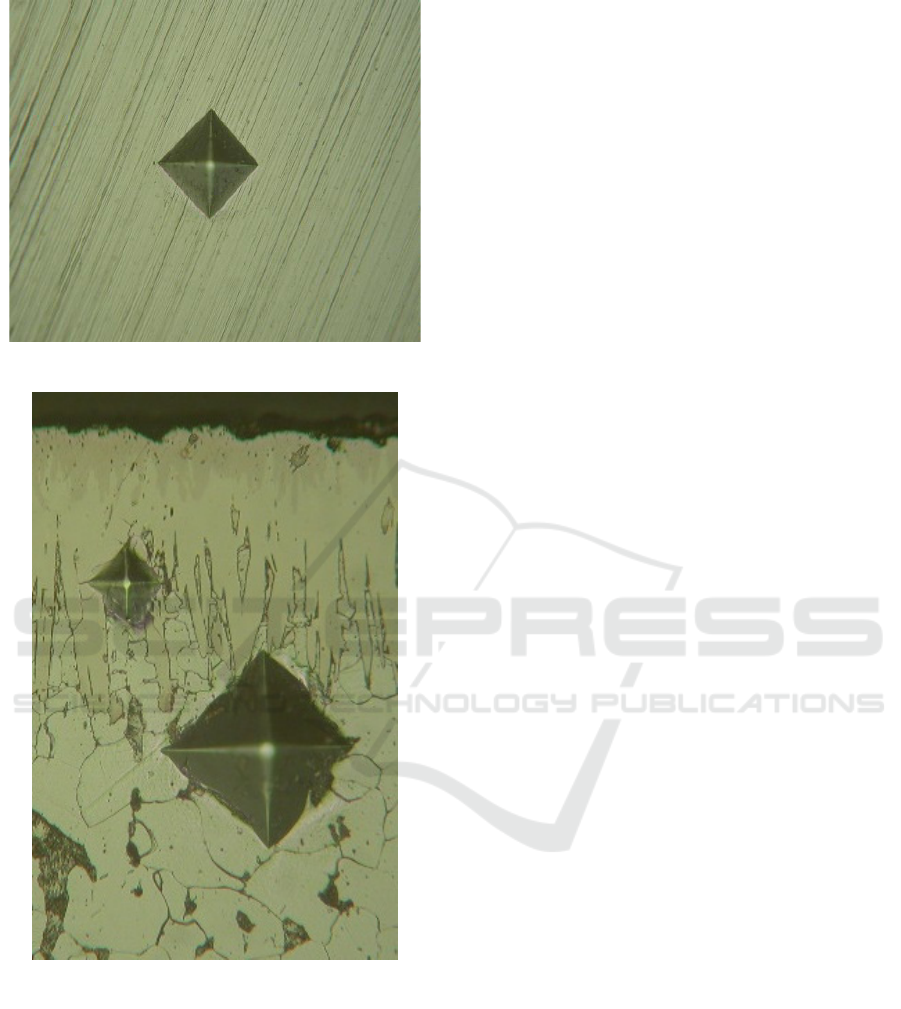
hardness using a LeitzMiniload test kit with a 5 kg
load. The indenter used is Vikers type. The vertical
pyramid indicator angle is 136 °. Giving style is
done slowly without collision. When touching the
indenter is held for 10 to 15 seconds. Styles are
given according to the load mounted on the test
equipment. After the force is removed, the track of
the indenter on the sample is measured. The material
hardness is calculated by equation (Setiawan, 2010):
Figure 3. The Vickers Hardness Instrument
Figure 4. The Vikers identor Leitz Miniload
HV = vikers micro hardness;
d = average identor spell;
P = given force
Micro hardness testing is performed on two
different points to see the gradationhardness of the
boride layer. The assumption in this test is at a
certain depth of valuethe violence is the same.To
determine the diffraction pattern and the atomic
position in the molecule the samplecharacterized by
XRD. From the obtained diffraction pattern can be
calculated size of crystallites (particle size). The
crystallite size can be calculated using the Debye
equation Scherrer as follows (Setiawan, 2010):
22
4.1854)2/sin(2000
d
P
d
aP
HV
ICRI 2018 - International Conference Recent Innovation
2948
CosL
K
hkl
hkl
Where:
K : form factor, was 0.9 for ball shape.
: X-ray wavelength (Å).
β
hkl
: FWHM (rad)
L
hkl
: crystal size (Å)
: diffraction angle.
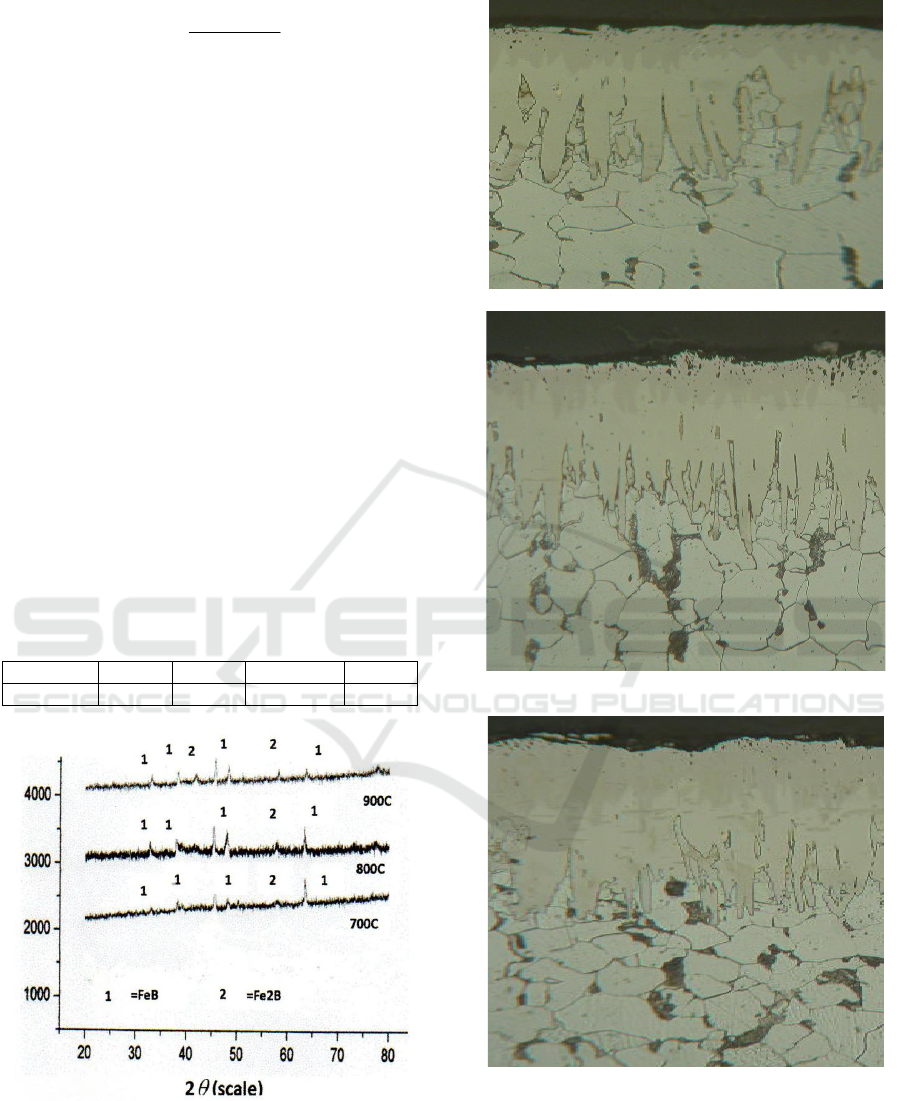
3 RESULT AND DISCUSSION
The basic material samples performed by initial
characterization are S45C which is a soft iron or
low carbon alloy steel. Both samples characterized
using XRF (X-ray flourence) to determine the
composition elements in the material and XRD to
know the phases and crystal structures. The
boronization powder used in this study is B4C,
SiC, and KBF
4
. After that the three powders are
mixed with each other so that obtained
boronizingpowder ready to use. The steps of
execution of sample characterization and
boronization powder are as follows.
Table 1. The composition in Steel S45C
Element C Si Mn P
S45C 0.45% 0.30% 0.6-0.9% 0.03%
Figure 5. XRD data of iron sample on the temperature of
700, 800, and 900
o
C.
a
b
c
Figure 6. View of boridedcross-sections treated at
(a)700
o
C, (b) 800
o
C, and (c) 900oC for 8 hours with
powder pack boriding thickness.
FeB
FeB
FeB
Fe2B
Fe2B
Fe2B
diffusion zone
diffusion zone
diffusion zone
Technology of Iron Hardening by using the Pressure and Heating Technique
2949
a
b
Figure 7. The hardness of iron S45C (a) before treatment
(b) after treatment
The results of characterization with XRD for the
S45C iron samples indicate Fe iron phases at certain
angles (figures 4). While the results of
characterization with XRF written in table 1 also
indicated the composition of carbon elements with
low concentration. This suggests that the S45C iron
samples are low carbon steels which are often used
as samples on the engineering of metal surface
hardening.
In order to obtain better results in the surface
hardening of the S45C iron samples, it is necessary
to minimize the particle size of the boronized
powder particles which will serve as the raw
material for metal hardening. The size of the particle
diameter can be obtained by milling (piercing) the
boronization powder within 10 hours. Boronized
powder is inserted into tubes containing iron balls of
different diameter sizes. Tubes that already contain
iron balls and boronisasi powder then vibrated or
rotated with a certain speed and time. From the
results of the milling obtained a decrease in particle
diameter size from 8.374 micron to 0.6885 micron
boronized powder.
The comparison of hardness S45C iron samples
between before and after boronization has increased.
The hardness on the iron surface S45C was 125 HV
before treatment and after boronization the hardness
increased to 887 HV.
4 CONCLUSION
From the sample preparation and characterization
results temporarily, it can be concluded:
1. With heating and pressure technologies applied to
powder boronization hardening methods can
increase the surface hardness of iron samples
S45C from 125 HV to 887 HV.
2. In terms of research operational costs, heating and
pressure techniques can save up to 60% when
compared to vacuum or argon gas drainage
techniques
REFERENCES
Anil Kumar Sinha, Boriding (Boronizing) of Steel, Bohn
Piston Division, ASM Handbook Volume 4, Heat
Treating (ASM International), 1990
Bintang Adjiantoro dan Romiyarso, T.B. (2005).
Karakterisasi Material Baja Karbon Rendah Hasil
Proses Boronisasi Padat, Jurnal Metalurgi, Vol.20,
No.1, 3 – 11
Dybkov, W Lengauer, P Gas, Formation of boride layers
at the Fe-25% Cr alloy-boron interface, J Mater
Sci (2006) 41:4948 – 4960
Gopalakrishnan, P. Shankar, M. Palaniappa, SS.
Ramakrishnan, Interrupted Boriding of Medium-
Carbon Steels, Metallurgical and Materials
Transactions A, Vol.33A, May 2002, 1475-1485
ICRI 2018 - International Conference Recent Innovation
2950

Martini, G. Palombarini, Mechanism of Thermochemical
Growth of Iron Borides on Iron, Journal of
Material Science 39 (2004) 933-937
Roumiana S. Petrova, N. Suwattananont, V.Samardzic,
The Effect of Boronizing on Metallic Alloys for
Automotive Applications, Journal Material
Engineering and Performance, Vol. 17(3) June
2008, 340-345
Sugondo, Langenati, R., Futichah, danMujtahid. (2010).
Pelapisan Nosel Roket dengan Boron Karbida, J.
Teknologi Bahan nuklir, Vol.6 No.1, 50-68
Smallman, R.E., Bishop, R.J. (1999). Metalurgi Fisik
Modern danRekayasa Material, Edisi IV,
Terjemahan oleh Ir. SriatiDjaprie, M. Met,
FakultasTeknik UI, Erlangga, Jakarta.
Setiawan, Analisis Lapisan Besi Borida pada ST37 dan
S45C yang diboronisasi dengan Teknik Powder
Pack, Universitas Indonesia, 2010
Suwattananont, Roumiana S. Petrova, James L Zunino,
Daniel P. Schmidt, Surface Treatment with Boron
for Corrosion Protection, Tri-Service Corrosion
Conference, Institute of Technology, Newark,
2005
Walter Fichtl, Boronizing and its Practical Applications,
Material in Engineering, Vol.2, December 1981,
hal. 276-2
Technology of Iron Hardening by using the Pressure and Heating Technique
2951
