
Impact of Environmental Factors of Water on Zooplankton Diversity
and Dynamic in Yacoub El Mansour Reservoir, Morocco
Ahlam Chakir* and Aicha Saadi
Laboratoire d’Hydrobiologie, Ecotoxicologie et Assainissement (LHEA)
Département de Biologie Faculté des Sciences Semlalia B.P. 2390 | 40000 Marrakech
Keywords: Copepods, Cladocerans, Zooplankton, Reservoir lake.
Abstract: The spatio-temporal distribution of crustacean zooplankton in relation to environmental factors was studied
in the Yacoub El Mansour reservoir in a semi-arid climate in the province of El Haouz, in Oued N'fis, located
at 65 km south of Marrakech (Morocco). The samples are taken during two annual cycles 2012 and 2013. In
this study, 6 species of crustacean zooplankton divided into 2 groups: copepods and cladocerans were
identified. Daphnia lumholtzi is the most dominant species, accounting for 58.26% of the total zooplankton.
The analysis of the results obtained at the studied reservoir shows a low zooplankton specific richness and a
great spatial heterogeneity. A canonical correspondence analysis (CCA) was used to estimate the influence of
environmental factors on the studied crustacean evolution. Thus, in the studied reservoir lake, hydrodynamics
of the ecosystem, trophic relationships and environmental factors are generally responsible for the spatial and
temporal distribution of these zooplankton species.
1. INTRODUCTION
The biodiversity of aquatic ecosystems is threatened
by hydrological dysfunctions, anthropogenic
pollution, habitat fragmentation, overexploitation of
some aquatic species, invasive organisms and by
climate change (Underwood et al. 2006). Databases
on "macroscopic diversity" such as birds, mammals
or higher vegetation have been produced. However,
data on "microscopic diversity", particularly those of
microfauna and algal microflora, are still fragmented,
especially in freshwater ecosystems. Moreover, in
recently watered reservoirs, the significant amount of
organic matter from the flooded immersion could
stimulate bacterial production and lead to a high
amount of heterotrophic and mixotrophic organisms.
This could be a source of crustacean zooplankton’s
food (Paterson 1997). Zooplankton plays a critical
role in aquatic food chains. It is an important source
of food for planktivorous fish and invertebrates. Also
it intensely grazes algae, bacteria, protozoa and others
invertebrates (Balvay 1990). Zooplankton
community responds rapidly to environmental
change because most species have very short-lived
generations. The study of these organisms remains a
necessity for developing effective strategies
for hydraulic and trophic resources management. In
Morocco, few hydrobiological studies have been
carried out on the zooplankton in reservoirs lakes sash
as: Lalla Takerkoust reservoir (Tifnouti 1993),
Hassan I reservoir (Benzekri 1992), El Kansra
reservoir (Fqih Berrada et al. 2000), The Mansour
Eddahbi reservoir (Sadani 2005) and on the level of
Zima and Sedd-El-Messjoun (Saadi 1994,
2002). This study of zooplankton was never carried
out in the Yacoub El Mansour reservoir, which
prompted us to study the interrelations of crustacean
zooplankton with the different physical and chemical
parameters of a recently watered reservoir under
semi-arid climate.
2. MATERIALS AND METHODS
2.1 Study Area
The Yacoub EL Mansour dam is located in N'fis’
river, 65 km south of Marrakesh city. It is about 20
km upstream Lalla Takerkoust dam and 1.5 km north
of Wigand village. Thanks to its reserve of 70 million
m3, the dam improves the regulation capacity of
N’fis’ river in Lalla Takerkoust dam. Also it
Chakir, A. and Saadi, A.
Impact of Environmental Factors of Water on Zooplankton Diversity and Dynamic in Yacoub El Mansour Reservoir, Morocco.
DOI: 10.5220/0009774403750382
In Proceedings of the 1st International Conference of Computer Science and Renewable Energies (ICCSRE 2018), pages 375-382
ISBN: 978-989-758-431-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
375

decreases water loss downstream. The dam is built
using concrete compacted with a ruler. It is 70 m
height with a crest length of 233 m. This dam help
increase the water volume of N'fis inputs, this volume
being 68-85 million cubic meters per year (Fig.1)
Figure 1: Geographical location on the Yacoub El Mansour dam at the Tensift El Haouz basin (Morocco.
2.2 Sampling
The measurement spots are distributed among several
stations in the same area and on several depths in each
station. In our study we analyzed two axes of
variation: the spatial axis and the temporal axis,
during two annual cycles from January 2012 to
December 2013. With a monthly sampling interval, in
autumn and winter and fortnightly in summer and
spring. The different sampling stations are as follow:
A dam station (S1): located at the bridge. At
this station, samples were taken at different
depths: Surface area, -1, -2.5, -5, -10, -15, -
19.5m, 1m of the bottom and the bottom
(Fig.1).
Littoral stations: (S2) located at the entrance
of the Ouirgane River and (S3) located at the
entrance of the N’fis River (Fig.1).
The water samples at the dam station (S1) were taken
using a closed bottle of the Van Dorn type with a 2L
capacity, while those from the littoral stations were
taken directly from the surface, with a 2L volume
sampler as well. The zooplankton were collected
using a plankton net of 50 cm in diameter and 50 μm
in mesh, collected in jars and fixed with 5% formalin.
Because of their low numbers, the zooplankton
species studied are counted on all samples in a Dolfus
tank. The count is done under a binocular magnifying
glass and species determination is made using the
determination keys (Dussart 1969) for the Copepods
and (Amoros 1984) for the Cladocerans.
2.3 Statistical Analysis
We determined the importance of the various
correlations between the zooplankton and physico-
chemical variables by the canonical correspondence
analysis CCA (Ter Braak, 1986), using XLSTAT
software.
3. RESULTS
Six zooplankton species were identified during the
period of our study: 4 for Cladocerans and 2 for
Copepods. The seasonal variations of the density
(ind/L) of these zooplankton species at the different
stations studied are represented in Figure 2.
The variation in zooplankton density shows a
spatio-temporal fluctuation. The biannual cycle is
marked by a maximum number of species in early
autumn of the first year (2012) and in spring during
the second year (2013). At the level of the Yacoub El
Mansour reservoir, the low specific richness affects
all the groups and more particularly that of the
copepods, which are found throughout the sampling
period but with a low abundance of 17.88% compared
to the total zooplankton. This group is represented by
Tropocyclops prasinus (Fischer, 1860) species, with
5.70% (considering the percentage represented by
each species in relation to the total number of
individuals), which appear to be more numerous in
2013 with a maximum number of individuals (78
ind/L) in April and less present in 2012 (Fig.3).
ICCSRE 2018 - International Conference of Computer Science and Renewable Energies
376
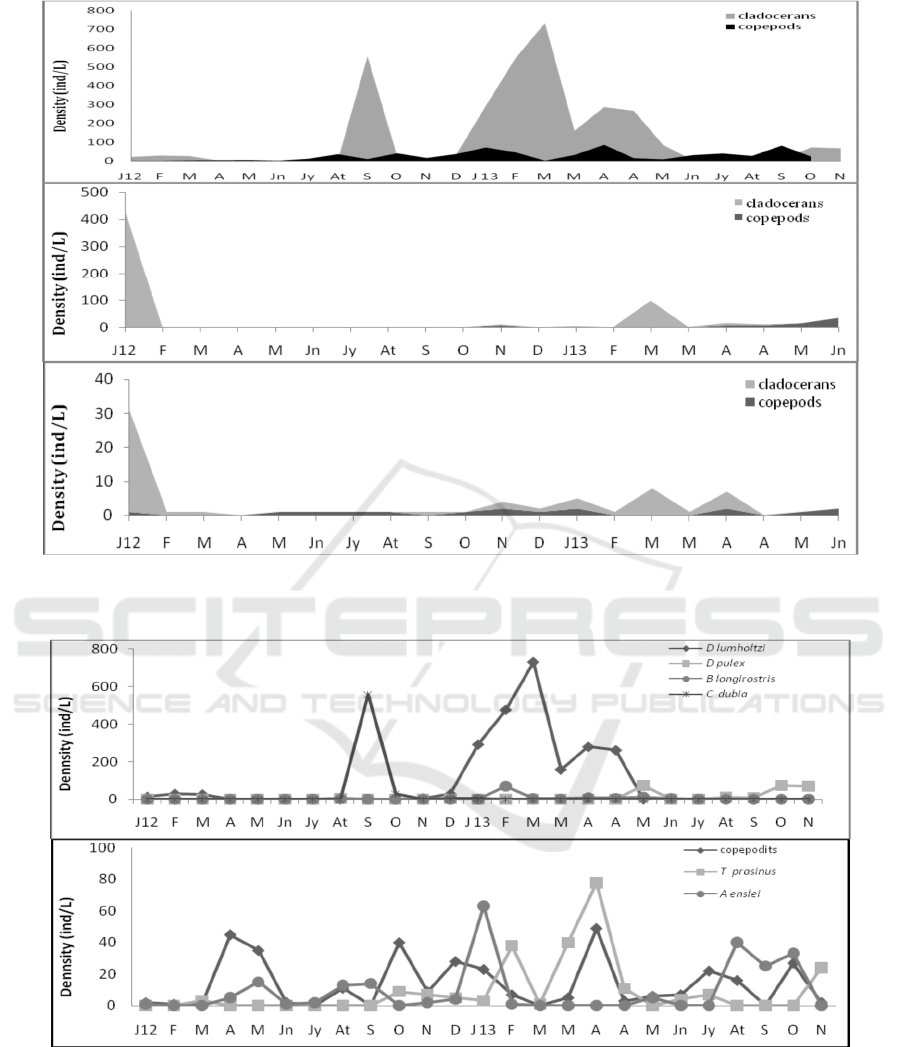
Figure 2: Temporal variations of zooplankton density at the dam station S1 (a) and at the two littoral stations S2 (b) and S3
(c).
Figure 3: Temporal variation of the different species of cladocerans density (a) and copepods density (b) at the dam station
(S1).
Whereas Acanthocyclops einslei
(MirabdulLayev & defay, 2004) species (5.56%)
reaches its maximum density in January 2013, with
63 ind/l. Concerning the copepodite stages, they are
present almost throughout the study period and
present 48.37% of the copepods and 8.65% of the
whole crustacean zooplankton (Fig.3). Generally the
settlement is dominated by the Cladocerans
(82.11%). The maximum peak of their density is
reached in September and March respectively for the
Impact of Environmental Factors of Water on Zooplankton Diversity and Dynamic in Yacoub El Mansour Reservoir, Morocco
377

year 2012 and 2013. Whereas during the first
sampling campaigns, the density of the settlement is
very low or null and the individuals are replaced by
resting eggs in S1 station. At the dam station (S1), the
seasonal succession of the different species of this
group (Fig. 3) presented an annual cycle
characterized by the dominance of the species
Daphnia lumholtzi (Sars, 1885) (58.26%), which is a
perennial species in this lake and has a maximum
development in spring (729 ind/L). It is followed by
Ceriodaphnia dubia (Richard, 1894) with an
abundance of 14.97%. This species reaches its
maximum (557 ind/L) in September, while the two
species Daphnia pulex (Leydig, 1860) and Bosmina
longirostris (O.F. Muller, 1785) have a low
abundance of 6.04% and 2.84%, respectively;
Daphnia pulex density increases towards the end of
spring and autumn with a maximum of 73 ind/L.
Whereas Bosmina longirostris shows a maximum
peak in February with 71 ind/L. At the two littoral
stations S1 and S2 (Fig. 4) the species Daphnia
lumholtzi is most present also with a maximum in
January 2012 of 430 ind/L at S2 and 30 ind/L at S3 .
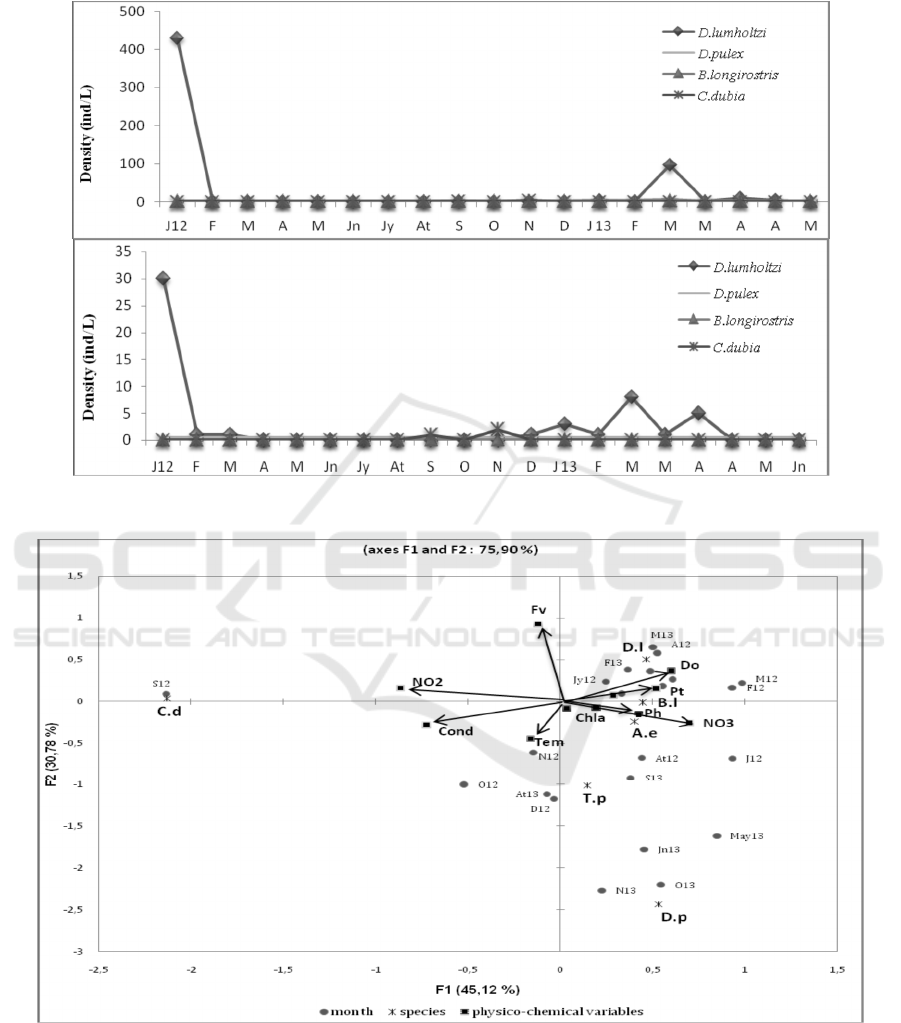
The influence of eleven physical and chemical
parameters (temperature, pH, conductivity, dissolved
oxygen, total phosphorus, orthophosphates, nitrites,
nitrates, ammonium and chlorophyll a) on the
different zooplanktonic species studied, at the
Yacoub El Mansour reservoir, was assessed using
Canonical Correspondence Analysis (CCA). The first
two axes of the CCA represent 45.12% and 30.78%
of the total inertia. The first factor axis (F1) has been
strongly associated with C.dubia (C.b) species,
conductivity (Cond) , dissolved oxygen (Do) and
nitrate (NO
3
), while the second factor axis (F2) is
strongly related to D.pulex (D.p) species, filling
volume (Fv) and Chlorophyll a (chl a). It is also noted
that, dissolved oxygen is negatively correlated with
temperature (Tem) and positively correlated with
NO
3
, total phosphorus (Pt) and orthophosphates
(PO
4
) .The species such as Daphnia lumholtzi and
Bosmina longirostris were associated with high value
of dissolved oxygen, total phosphorus (Pt) and
orthophosphates (PO
4
), during the months of
February – April 2012, whereas species such as
Daphnia pulex, Acanthocyclops einslei and
Tropocyclops prasinus were associated with high
values for pH, nitrates and chl a. while, Ceriodaphnia
dubia species is associated with highest volume of
filling (Fv) and nitrites (NO
2
). In CCA ordination
diagram, Ceriodaphnia dubia, a cladoceran species,
occupies an aberrant position due to its occurrence
only in September 2012 when a substantial increase
in NO
2
levels was evident (Fig. 5).
4. DISCUSSION
The abundance and specific zooplankton richness in
the Yacoub El Mansour reservoir are low compared
to those found in other Moroccan dams; 8 crustaceans
zooplankton at the Lalla Takerkoust reservoir
(Tifnouti 1993) and 12 at the Hassan I reservoir
(Benzekri 1992). Indeed, the Yacoub El Mansour
reservoir was recently put into water. Also, the period
of study coincides with a period of frequent draining
especially during the year 2012. This generates a
short retention time in the reservoir (Benzha 2005).
The impact of draining on the zooplankton
populations was underlined by many authors
(Axelson 1961, Rodhe 1964, Pechlaner 1964). Brook
and Woodward (1956) found that high rates of water
turnover can involve quantitative and qualitative
variations of the plankton in the lakes. In general, the
low abundance of Cladocerans and Copepods is
associated with environmental conditions, caused by,
the hydrodynamics of the reservoir, such as the low
water volume, short residence time and morphometry
(Isumbisho 2006). Predation by planktivorous fish
and the poor availability of food sources, may also
lead to a reduction in the specific richness of the
reservoir (Achembach and Lampert 1997). According
to an earlier study on phytoplankton at Yacoub El
Mansour reservoir (Hammou 2014), the
phytoplankton population inventoried in the Yacoub
El Mansour reservoir is not very diversified, and
quantitatively only a few species play a decisive role
in this lake. This may also explain the low specific
richness of zooplankton communities at the reservoir.
It was found that at the Yacoub El Mansour reservoir,
the arid climate favored the existence of two periods
of abundance during the year: an autumn-spring
period favorable to the development of zooplankton
and a winter-summer period, characterized by a
significant decline of the number of species, their
densities and their distributions. During the rainy
season, water supplies from the reservoir upstream
and precipitation tend to cause small mixtures of
water bodies, nutrients are then available in the mass
of oxygenated water and are very rapidly assimilated
by the invertebrates which could lead to the
development of zooplankton. In addition, some fish
take advantage of exogenous inputs during the rainy
season. Whereas in the dry season some fish consume
endogenous material (mainly micro-crustaceans) and
consequently cause a decrease in the abundance of the
zooplankton population (Horeau et al. 1998).
Muylaert (2003) also corroborated the conclusion,
that zooplankton biomass generally reaches its peak
during rainfall in reservoirs. In spring, physical
ICCSRE 2018 - International Conference of Computer Science and Renewable Energies
378
factors (nutrient input, photoperiod and temperature
increase) increase the primary production
(phytoplankton). Which increase zooplankton density
(Tifnouti 1993).
Figure 4: Temporal variation of the different species of cladocerans density at the littoral stations S2 (a) and S3 (b).
Figure 5: CCA ordination diagram with zooplankton species and environmental variables in Yacoub El Mansour reservoir.
The zooplankton species shown are: Daphnia lumholtzi (D.l), Ceriodaphnia dubia (C.d), Daphnia pulex (D.p), Bosmina
longirostris (B.l). The environmental variables are: filling volume (Fv), total phosphorus (Pt), Orthophosphates (PO4), nitrites
(NO2), nitrates (NO3), ammonium (NH4), chlorophyll a (Chl), dissolved oxygen (Do), conductivity (Cond), Temperature
(Temp) and pH. The Months are :January 2012 or 2013 (J12 or 13), February (F), March (M), April (A), June (Jn), July(Jy),
August (At), September (S), October (O), November (N), December (D).
Impact of Environmental Factors of Water on Zooplankton Diversity and Dynamic in Yacoub El Mansour Reservoir, Morocco
379

Positive correlations between pH and conductivity
with some zooplanktonic species (Fig. 5) show that
alkaline pH and high conductivity also promote the
growth of some zooplankton in the dam reservoir.
This is in agreement with the conclusions of Byars
(1960), Hujare (2005) and Mustapha (2009). At the
dam station (S1), the density of the zooplankton
species is higher compared to the two littoral stations
S2 and S3, due to their relative stability and slower
flow velocity. Similar results were found by Tifnouti
(1993), at the Lalla Takerkoust reservoir. In the
summer according to the PEG model (Plankton
Ecology Group), small Cladocerans are replaced by
larger Cladocerans and adults Copepods (Sommer et
al. 1986, Lair and Ayadi 1989, Tifnouti 1993). In the
Yacoub El Mansour reservoir, during the summer,
there was an absence of most crustacean zooplankton
and a presence of a few individuals of Copepods and
Cladocerans, mainly the species B.longirostris, a
species of small size, which continues its
development by parthenogenesis until late spring.
The population passes through a sexual reproduction
around June, which ensures the appearance of a new
generation next winter. This type of development is
similar to that observed by Tifnouti (1993), at the
level of Lalla Takerkoust reservoir. At the Yacoub El
Mansour reservoir this species reaches its maximum
density in February, which is in agreement with the
results of Vijverberg (1980). Which, considers that
the development of the species in question is adapted
to the low temperatures of the environment. The study
of the vertical distribution of zooplankton shows that
the zooplankton is concentrated over the first 10
meters. The depletion of zooplankton, particularly at
young stages, in depth from -15 m, may be related to
the high suspended mater (Tifnouti 1993), or to low
availability of food. Juvenile stages preferentially
stay in warmer and more nutritious surface waters
(Hutchinson 1967, Kerfoot 1980).
5. CONCLUSION
In conclusion, zooplankton settlement in the Yacoub
El Mansour oligotrophic lake (Chakir and Saadi
2016), set in 2008 is characterized, by a very low
number of individuals per liter and a low specific
richness (4 Cladocerans and 2 Copepods). The spatial
and temporal variations of the various zooplankton
species follow a distribution pattern strongly
influenced by conditions. Which fluctuate according
to the season: water level, temperature, pH,
conductivity, dissolved oxygen, suspended matter,
total phosphorus, orthophosphates, nitrites, nitrates,
ammonium, and Chlorophyll a. Moreover, the floods
of the wet season favor the appearance of sporadic
species. Among all the factors studied, the
fluctuations in water level and temperature associated
with the semi-arid climate of the region, presenting a
period of great drought, would be tow of the main
causes of the temporal distribution of the species in
the Yacoub El Mansour reservoir. This study should
be completed taking into account the other
components of the trophic chain, in particular
Protozoa, Rotiferes, Phytoplankton and fish, in order
to integrate the "crustacean zooplankton" component
into the conceptual models that describe the effect of
the manipulations of trophic chains on water quality.
Generally, successive drainings should be taken into
account in the management of the tanks where fish
farming is required.
ACKNOWLEDGEMENTS
We would like to express our deepest thanks to Mds
Defaye D., for the determination of the species
Acanthocyclops einslei and Ms Lachir A., for their
invaluable assistance in the translation for this
manuscript.
REFERENCES
Achembach L., Lampert W. 1997. Effects of elevated
temperatures on threshold food concentrations and
possible competitive abilities of differently sized
cladoceran species. Oikos 79: 469-476.
Amoros C. 1984. Crustacés cladocères: Introduction
pratique à la systématique des organismes des eaux
continentales françaises. Bulletin mensuel de la Société
Linnéenne de Lyon 53e année, (3).
Axelson J. 1961. Zooplankton and impoundment of two
lakes in Northern Sweden (Ransaren and
Kultsjön). Rept. Inst. Freshw. Res. Drottningholm, 42,
84-168.
Badsi H., Ali H. O., Loudiki M, El Hafa M, Chakli R, &
Aamiri A. 2010. Ecological factors affecting the
distribution of zooplankton community in the Massa
Lagoon (Southern Morocco). African Journal of
Environmental Science and Technology, 4(11), 751-
762.
Balvay G., Gawler M., Pelletier J. P. 1990. Lake trophic
status and the development of the clear- water phase in
Lake Geneva. In Large Lake. Springer Berlin
Heidelberg. 580-591.
Benzekri M. A. 1992. Qualité des eaux du lac réservoir
Hassan I (Maroc): Hydrochimie et dynamique
pluriannuelle du zooplancton. Thèse de Doctorat Univ.
Cadi Ayyad Marrakech.
ICCSRE 2018 - International Conference of Computer Science and Renewable Energies
380

Benzha F. et al. 2005. Qualité physico-chimique des eaux
du réservoir Daourat; impact de la vidange sur son
fonctionnement. Revue des sciences de l'eau/Journal of
Water Science, 18. 57-74.
Brett M. T. 1989. Zooplankton communities and
acidification processes (a review). Water, Air, and Soil
Pollution, 44, 387-41
Brook A. J., Woodward W.B. 1956. Some observations on
the effects of water inflow and outflow on the plankton
of small lakes. J. Anim. Ecoi, 25: 22-35.
Byars J.A. 1960. A Freshwater Pond in Zew Zealand.
Marine and Freshwater Research, 11(2), 222-240.
Chakir A., Saadi A. 2016. Water quality of a recent lake
reservoir in a semi-arid climate; Yacoub El Mansour
(Morocco). Journal of Environment and Earth Science
www.iiste.org. l6, (9), 217-228.
Chaparro G., Marinone M. C., Lombardo R. J, Schiaffino
M R, de Souza Guimarães A, O’Farrell I. 2011.
Zooplankton succession during extraordinary drought–
flood cycles: a case study in a South American
floodplain lake. Limnologica-Ecology and
Management of Inland Waters, 41(4), 371-381.
Dodson S. 1992. Predicting crustacean zooplankton species
richness. Limnology and Oceanography 37, 848-856.
Dussart B. 1967. Les Copépodes des eaux continentales
d'Europe occidentale. Tome I : Calanoïdes et
Harpacticoïdes. Boubée N. éd., Paris, 500 pp.
Dussart B. 1969. Les Copépodes des eaux continentales
d'Europe occidentale. Tome II : Cyclopoïdes et
Biologie. Boubée N. éd., Paris, 292 pp.
Fqih Berrada D., Berrada R., Benzekri A., Fahde A. 2000.
Hétérogénéité horizontale des peuplements
microphytoplanctoniques et zooplanctoniques en
relation avec les paramètres abiotiques dans la retenue
El Kansera (Maroc). Revue des sciences de
l'eau/Journal of Water Science, 13(3), 213-236.
Hammou H. A., Latour D., Sabart M., Samoudi S., Mouhri
K., Robin J. & Loudiki M. 2014. Temporal evolution
and vertical stratification of Microcystis toxic potential
during a first bloom event. Aquatic ecology, 48(2), 219-
228.
Horeau V., Richard S. & Cerdan P. 1998. La qualité de l'eau
et son incidence sur la biodiversité: l'exemple de la
retenue de Petit Saut (Guyane française). Journal
d'agriculture traditionnelle et de botanique appliquée,
40(1), 53-77
Hujare M. S. 2005. Hydrobiologial studies on some water
reservoirs of Hatkanangale Tahsil (Maharashtra)
(Doctoral dissertation, Thesis, Shivaji University,
Kolhapur).
Isumbisho M., Sarmento H., Kaningini B., Micha J. C.,
Descy J. P. 2006. Zooplankton of Lake Kivu, East
Africa, half a century after the Tanganyika sardine
introduction. Journal of Plankton Research, 28(11),
971-989.
Keller W., Yan N. D. 1991. Recovery of crustacean
zooplankton species richness in Sudbury area lakes
following water quality improvements. Journal
canadien des sciences halieutiques et aquatiques 48,
1635-1644.
Mieczan T., Adamczuk M., Tarkowska-Kukuryk M.,
Nawrot D. 2015. Effect of water chemistry on
zooplanktonic and microbial communities across
freshwater ecotones in different macrophyte-dominated
shallow lakes. Journal of Limnology, 75(2).
Mirabdullayev I. M., Defaye D. 2004. On the taxonomy of
the Acanthocyclops robustus species-complex
(Copepoda, Cyclopidae): Acanthocyclops
brevispinosus and A. einslei sp. n. Vestnik Zoologii, 38,
pp. 27–37.
Mustapha M. K. 2009. Zooplankton assemblage of Oyun
reservoir, offa, Nigeria. Revista de biologia tropical,
57(4), 1027-1047.
Muylaert K., Declerck S., Geenens V., Van Wichelen J.,
Degans H., Vandekerkhove J., Urrutia R. 2003.
Zooplankton, phytoplankton and the microbial food
web in two turbid and two clearwater shallow lakes in
Belgium. Aquatic Ecology, 37(2), 137-150.
Pace M. L. 1986. An empirical analysis of zooplankton size
structure across lake trophic gradients. Limnology and
Oceanograph 31, 45-55.
Paterson M. J., Findly D., Beaty v., Findly W., Scindler E.
U., Stainton M., Mccullough G. 1997. Changes in the
planktonic food web of new experimental reservoir,
Can. J. Aquat. Sci. 54(1997) 1088–1102.
Paterson M. 2014. Protocoles du réseau d'évaluation et de
surveillance écologiques (résé) pour mesurer la
biodiversité : zooplancton des eaux douces ministère
des pêches et des océans institut des eaux douces 501,
university crescent winnipeg (manitoba)
r3t 2n6.
Rey J. 1988. Étude comparée de la dynamique du
zooplancton de trois réservoirs d'altitude et d'un lac
naturel dans les Pyrénées. Annales de limnologie, 24,
No. 2, pp. 139-160. EDP Sciences.
Saadi A., Champeau A. 1994. Density, Biomass and
production of Arctodiaptomus salinus (Copepoda,
Calanoida) in a temporary brackish hydrosystem, the
Zima Sebkha (Morocco). Ecologie, t.25(2) : 71-78.
Saadi A., Champeau A. 2002. Hydrobiologie de deux
hydrosystèmes temporaires saumâtres : Zima et Sedd-
El-Messjoun (bassin de la Bahira, Maroc). Revue des
Sciences de L’eau, 6(3) : 319-33.
Sadani. 2005. Impact des sources de pollution sur la qualité
des ressources en eau en milieu aride: cas du bassin
versant du lac de barrage Mansour Eddahbi, Thèse 3
éme cycle, Univ. Cadi. Ayyad, Fac. Sci., Marrakech,
175 p.
Sommer U., Gliwicz Z. M., Lampert W., Duncan A. 1986.
The PEG-model of seasonal succession of planktonic
events in fresh waters. Arch. Hydrobiol, 106 (4), 433-
471.
Tifnouti A. 1993. Structure et organisation du peuplement
zooplanctonique dans le lac réservoir Lalla Takerkoust.
Th. Doct. d'Etat, Univ. Cadi Ayyad, Marrakech, 268 p.
Ter Braak C. J. 1986. Canonical correspondence analysis: a
new eigenvector technique for multivariate direct
gradient analysis. Ecology, 67(5), 1167-1179.
Underwood E. C., Mulitsch M. J., Greenberg J. A., Whiting
M. L., Ustin S. L., Kefauver S. C. 2006. Mapping
Impact of Environmental Factors of Water on Zooplankton Diversity and Dynamic in Yacoub El Mansour Reservoir, Morocco
381

invasive aquatic vegetation in the Sacramento-San
Joaquin Delta using hyperspectral imagery. Environ
Monit Assess. 121, 47-64.
ICCSRE 2018 - International Conference of Computer Science and Renewable Energies
382
