
Recovery of an Estuarine Ecosystem after the Stopping of
Wastewater Discharges: Macrobenthic Community Characterization
in the Estuary of Oued Souss (Southwestern Morocco)
H. Bergayou
1
, E. Anajjar
1
, L. Lefrère
1
, A. Moukrim
1
, E. Gorman
2
and P. Gillet
2
1
Laboratory Aquatic Systems: Marine and Continental Environment (AQUAMAR), Team Biology, Ecology and
Development of Marine Resources , Faculty of Sciences, Ibn Zohr University, B.P. 8106, 80000, Agadir, Morocco
2
Center for Studies and Research on Aquatic Ecosystems, Institute of Applied Ecology, UCO, BP 808, 44, rue Rabelais,
49008 Angers 01, France
pgillet@uco.fr
Keywords: Biomass, Estuary, Macrobenthos, Macrofaunal assemblage, Specific richness, Wastewater.
Abstract: The communities of benthic macroinvertebrates, living in the estuary of Oued Souss (Agadir Bay,
Morocco), were studied in parallel with the changes that this ecosystem had underwent after the
stopping of pollution caused by untreated wastewater discharges. The specific richness was greater
in the year following the end of discharges (22 species in 2003 instead of 14 found during the
pollution period (2001-2002). A similar finding was noted for the dominance of the species.
Indeed, if the dominating species was Hydrobia ulvae during the pollution period, followed by
Hediste diversicolor and Scrobicularia plana in decreasing order, the sequence: H. diversicolor
H. ulvae Cerastoderma edule S. plana, was noted in 2003. The longitudinal distribution of
species living in this site in 2001 and 2002 had wider in 2003 and average biomass, determined by
the study of the ash-free dry weight, had become clearly greater.
1. INTRODUCTION
Estuarine ecosystems are part of most productive
coastal environments but remain the most vulnerable
due to exposure to toxic anthropogenic effluents
transported by rivers from remote and nearby
conurbations, and industrial and agricultural areas.
The estuary of the Oued Souss (30°21'N, 9°35'W),
located in an arid zone and part of the Souss-Massa
National Park in the Ramsar site, constitutes one of
the rarest humid areas in South Western Morocco. It
is an ecosystem of great ecological interest,
particularly for many migratory birds (Dakki et al.,
1995; El Bekkay, 2013; Oubrou & El Bekkay, 2014).
However, the estuary experienced a profound
ecological change. For a long time, it has been
subjected to the discharge of large amounts of sewage
and industrial effluents (Moukrim et al., 2000). And
since November 2002, the establishment of a sewage
treatment plant marked the end of wastewater
discharge in the estuary (Ait Alla et al., 2006).
If the period in which the estuary was receiving
the discharges has been the subject of several studies
(Snoussi, 1988; Id-Halla et al., 1998; Mimouni et al.,
2002; El Hamidi et al., 2002; Gillet et al., 2003;
Bergayou et al., 2005; Ait Alla et al., 2006a; Ait Alla
et al., 2006b; Anajjar et al., 2008; Moukrim et al.,
2008; Bergayou et al., 2008; Bergayou et al., 2009],
investigations after the establishment of the plant are
of interest because they allow not only to compare the
situation of the mouth of the valley before the end of
the wastewater discharges, but also to follow the
evolution of the restoration of the ecosystem.
It is within this framework that our laboratory has
set up a multidisciplinary research program covering
all the components of the ecosystem. Thus, the
research focused on the physico-chemistry of water
and sediment (Lefrère, 2005), heavy metals (Anajjar
330
Bergayou, H., Anajjar, E., Lefrère, L., Moukrim, A., Gorman, E. and Gillet, P.
Recovery of an Estuarine Ecosystem after the Stopping of Wastewater Discharges: Macrobenthic Community Characterization in the Estuary of Oued Souss (Southwestern Morocco).
DOI: 10.5220/0009771803300341
In Proceedings of the 1st International Conference of Computer Science and Renewable Energies (ICCSRE 2018), pages 330-341
ISBN: 978-989-758-431-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

et al., 2008; Moukrim et al., 2008), pesticides
(Agnaou et al., 2014), biomarkers (Ait Alla et al.,
2006a; Idardare, 2005; Bergayou et al., 2009),
population dynamics and the biology of some species
(Ait Alla et al., 2006b; Lefrere, 2005; Bergayou et al.,
2008).
It is within the framework of this research
program that our investigations are being conducted.
The aim is to study the benthic invertebrate
communities of the intertidal zone of the estuary
during the wastewater discharge period (2001, 2002)
and after their termination (2003). The analysis
focused on faunal composition, longitudinal
distribution of species, species richness, abundance,
dominance and biomass. It also made it possible to
define and compare the biocenotic units during the
two periods.
2. MATERIALS AND METHODS
Three campaigns were undertaken during the
summer season. Two of them were carried out while
the estuary was receiving wastewater discharge in
2001 and 2002. The 2003 campaign was carried out
after the cessation of the pollution. To ensure a good
coverage of the ecosystem through our samples, 12
radials along the estuary and part of Oued Souss are
defined. Radials were numbered from 0 to 12 from
the mouth to upstream with radial 7 being the direct
receptacle of wastewater during the discharge period
(Figure 1).
Figure 1. Location of the sampling stations of the
intertidal macrobenthos in Oued Souss estuary
(Agadir Bay, Morocco) during the summer
campaigns in 2001, 2002, and 2003.
Downstream from radial 2, the stations 1bis and
2bis belong to an arm of the Oued. Between radial 2
and radial 6, the mudflat is wide, and the bed of the
Oued shows sandbanks which, very often, are
accessible. This is why we generally chose four
sampling points by radial: two on the north bank (A,
A') and two on the south bank (B, B'). From radial 6
going upstream, the mudflat decreases. Therefore, we
selected only two points of sampling by radial: one on
each bank. A total of 36 and 39 stations were
respectively sampled at low tide during the period
before and after the discharges were terminated. Their
coordinates were recorded using a GPS (MLR brand,
type SP12X). During the last campaign, three
additional stations were selected (0bis A, 0bis B, and
2'). They are the result of marine hydrodynamics that
have made new sandbanks appear. Finally, it should
be noted that after the discharges stopped, there was
a modification of the watercourse that no longer
reached radial 7. Samples were taken using the
quadratic sampling technique (Elliot & Descamps,
1973). For all the stations considered, each sampling
point consists of 4 quadrats of 0.25m x 0.25m (over a
depth of 0.20m), ie a sampling area of 0.25m² per
sample.
The sorting of the different species was carried out
on site or in the laboratory, with a sieve with 1 mm
mesh. The animals were kept in ethanol at 70° and
their determination was carried out using a binocular
magnifier and, in some cases, using a microscope (to
study the morphology of the parapodia of some
Annelids).
As a first step, the inventory of fauna, the spatial
distribution of species, and parameters such as
abundance (number of individuals of a species in a
sample); the dominance (percentage represented by a
species, a class, or an embranchment in a sample) that
make it possible to reconstruct the faunistic
composition are determined. Species richness
(number of species present in the settlement) as well
as biomass were also evaluated. For the last
parameter, we performed a prior decalcification to the
molluscs in a 10% hydrochloric acid bath and a
drying at 80°C of all the individuals during 48 hours
(to measure the dry weight). The animals are then
placed in an oven at 600°C for 2 hours to reduce them
to ashes. The difference between the decalcified dry
weight and the ash quantity corresponds to the dry
weight of the soft masses (ashless), expressed in g/m²
(Bachelet et al., 1981).
In order to define biocenotic units, a hierarchical
ascending classification is performed. For this, we
first calculated the coenotic affinity between the
station communities estimated from the Jaccard
coefficient (Gillet, 1986):
J = Na, b / (Na + Nb - Na, b)
with Na: number of species in sample survey a, Nb:
number of species in sample survey b, Na, b: number
of species common to sample surveys: a and b. The
Recovery of an Estuarine Ecosystem after the Stopping of Wastewater Discharges: Macrobenthic Community Characterization in the
Estuary of Oued Souss (Southwestern Morocco)
331
different values are grouped in a similarity matrix
from which the dendogram is built according to the
algorithm (Lance & Williams, 1967):
dhij = 0.625 dhi + 0.625 dhj - 0.25 dij
This method is highly recommended by various
authors (Legendre & Legendre, 1998)
3. RESULTS
3. 1. Settlement Faunistic Composition
3.1.1. The Ecosystem Receiving Wastewater
Discharges
The macrobenthic fauna shows a similar faunal
composition for both seasons during the period when
the ecosystem was receiving wastewater discharges.
The number of individuals, all species combined,
amounts to 11270 and 9131 for 2001 and 2002,
respectively (Tables 1 and 2).
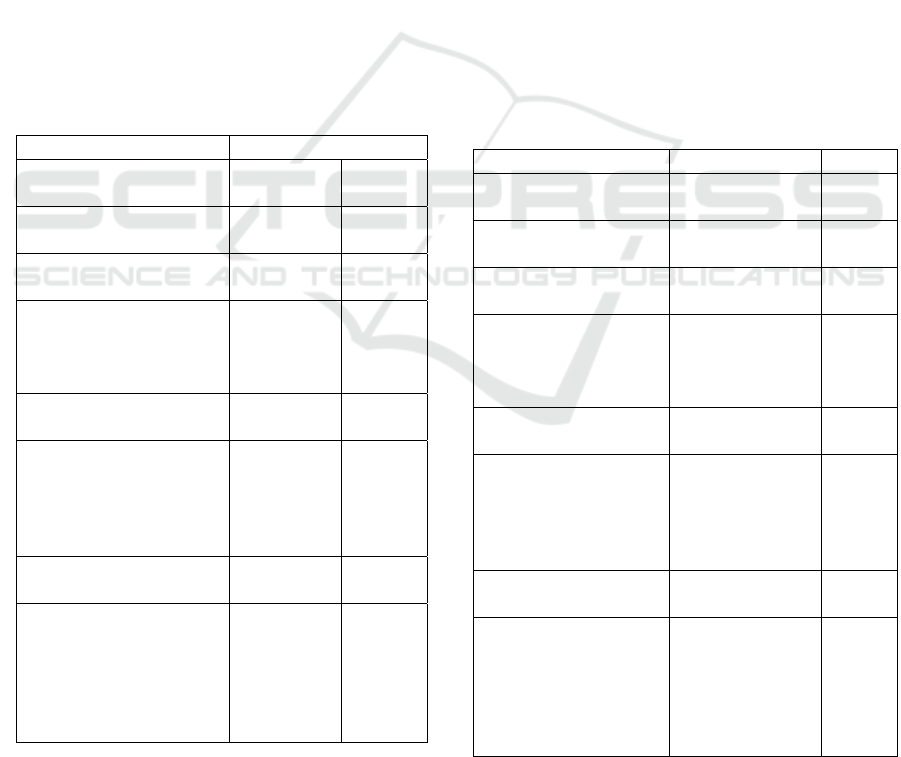
Table 1. Faunistic composition of the
macrobenthos of the Oued Souss estuary by phylum
during the period of wastewater discharges (in 2001).
2001 Campaign
Total
Abundance
%
Nemathelminthes
Sp
Annelids
2459 21.82
Arenicola marina
Glycera tridactyla
Hediste diversicolor
Pectinaria koreni
12
2426
21
0.106
21.526
0.186
Molluscs
8747 77.61
Cerastoderma edule
Hydrobia ulvae
Macoma cumana
Scrobicularia plana
378
6500
33
1836
3.354
57.675
0.293
16.291
Crustaceans
64 0.57
Bathyporeia sp
Carcinus maenas
Eurydice pulchra
Haustorius arenarius
Urothoe brevicornis
4
44
15
1
0.035
0,390
0.133
0.009
Molluscan phylum largely dominates the
settlement with 77.6% (in 2001) and 84.1% (in 2002).
The rest of the benthic fauna is divided between the
Annelids (21.82% in 2001 and 12.42% in 2002), the
Crustaceans (0.57% in 2001 and 3.18% in 2002) and
the Nemathelminthes which constitute a minority and
are identified only in 2002 (less than 1%).
The ecosystem is poor in terms of biodiversity
(table1). Only a limited number of species (N = 14)
are encountered. However, these species are
abundant. Thus, the annelid Hediste diversicolor and
the molluscs Hydrobia ulvae and Scrobicularia
plana, alone represent more than 94.5%. In fact, in
descending order, H. ulvae has an abundance of about
6500 and 6730 and densities of up to 7016 ind/m
2
and
8088 ind/m
2
respectively for 2001 and 2002; followed
by H. diversicolor with abundances of 2426 and 1112
and densities of up to 1464 ind/m
2
and 732 ind/m
2
respectively in 2001 and 2002; then S. plana with
abundances of the order of 1836 and 827 and densities
up to 3780 ind/m
2
and 1808 ind/m
2
respectively in
2001 and 2002.
Table 2. Faunistic composition of the macrobenthos
of the Oued Souss estuary by phylum during the
period of wastewater discharges (in 2002).
2002 Campaign
Total
Abundance
%
Nemathelminthes
Sp
25
0.274
Annelids
1134 12.42
Arenicola marina
Glycera tridactyla
Hediste diversicolor
Pectinaria koreni
12
10
1112
0.131
0.110
12.178
Molluscs
7682 84.13
Cerastoderma edule
Hydrobia ulvae
Macoma cumana
Scrobicularia plana
72
6730
53
827
0.789
73.70
0.580
9.057
Crustaceans
290 3.18
Bathyporeia sp
Carcinus maenas
Eurydice pulchra
Haustorius arenarius
Urothoe brevicornis
48
6
236
0.526
0.066
2.585
ICCSRE 2018 - International Conference of Computer Science and Renewable Energies
332
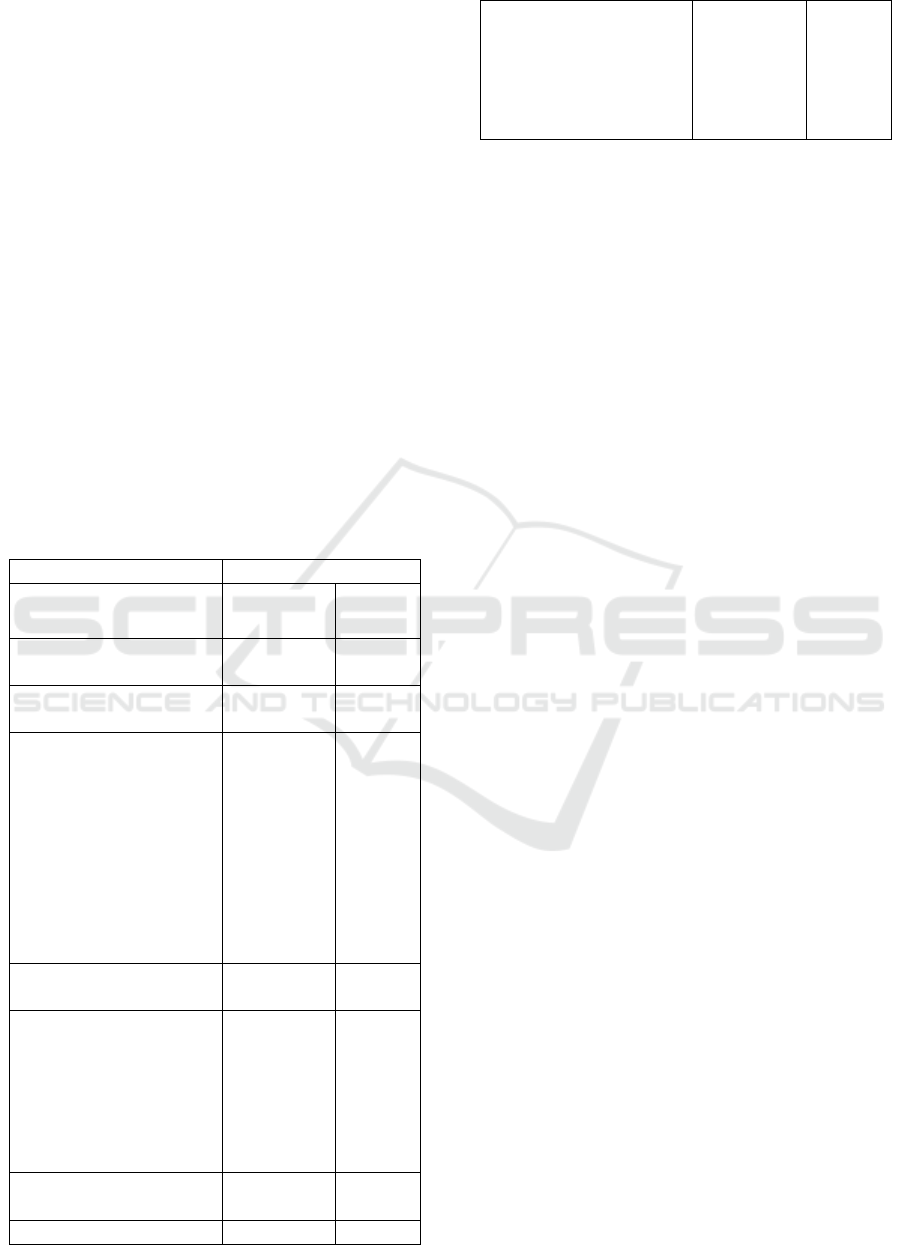
3.1.2. The Ecosystem after Stopping
Wastewater Discharges
The number of individuals harvested is of the order of
15968 (Table 3). There are 22 species belonging to
four phyla. The Molluscan phylum is dominant
(69.6%), followed by that of the Annelids (28.9%),
followed by the Crustacea (1.4%). Dominance by
species shows that it is Hediste diversicolor which
dominates the settlement with an abundance of 4599
individuals and a density which reaches in some
stations 2328 ind/m
2
, followed by Hydrobia ulvae
with an abundance of 4495 and a density of up to
4200 ind/m
2
, Cerastoderma edule with an abundance
of 3765 and a density of 6760 ind/m
2
and
Scrobicularia plana with an abundance of 2795 and a
density of up to 2336 ind/m
2
.
Table 3. Faunistic composition of the macrobenthos
of the estuary of Oued Souss by phylum after
cessation of wastewater discharges (2003)
2003 Campaign
Total
abundance
%
Nemathelminthes
Sp
16
0.100
Annelids
4615
28.9
Nemerte sp
Arenicola marina
Capitella capitata
Glycera tridactyla
Heteromastus filiformis
Hediste diversicolor
Lanice conchylega
Nephtys hombergii
Pectinaria koreni
1
5
1
1
1
4599
1
1
5
0.006
0.031
0.006
0.006
0.006
28.801
0.006
0.006
0.031
Molluscs
11113
69.6
Aplysia punctata
Cerastoderma edule
Donax trunculus
Hydrobia ulvae
Macoma cumana
Scrobicularia plana
2
3765
21
4495
35
2795
0.013
23.578
0.132
28.15
0.219
17.504
Crustaceans
224
1.4
Bathyporeia sp 48 0.301
Carcinus maenas
Eurydice pulchra
Gammarus marinus
Haustorius arenarius
Urothoe brevicornis
17
128
12
10
9
0.106
0.802
0.075
0.063
0.056
These data show that the ecosystem is
experiencing a significant ecological change in
faunistic composition. The number of individuals
harvested is significantly larger compared to the
period when the ecosystem received wastewater.
Although the phylum of molluscs is still dominant,
followed by that of Annelids and Crustaceans, the
composition of macrobenthic fauna and the
abundance of these organisms change during this
campaign carried out after the cessation of discards.
In fact, we notice an enrichment of the
macrobenthic population in new species and if we
consider the dominance of the species in decreasing
order, four species dominate the population, in
descending order: H. diversicolor, H. ulvae, C. edule
and S. plana instead of three species during the
discard period: H. ulvae, H. diversicolor and S. plana.
3. 2. Longitudinal Distribution of Species
and Biocoenotic Units
3.2.1. The Ecosystem Receiving Wastewater
Discharges
Species populating the environment were
divided into four groups of species, depending on
their distance from the sea (Figure 2):
- a first group linked to the mouth of the
estuary (radials 0, 1, 1bis) and not sinking beyond 250
meters. It consists of the following species: Arenicola
marina, Glycera tridactyla, Bathyporeia sp, Eurydice
pulchra, Haustorius arenarius and Urothoe
brevicornis;
- a second group that is distributed on radials
1, 1bis, 2bis and 2. It is Cerastoderma edule and
Macoma cumana;
- a third group corresponding to species with
very wide distribution on the estuary. It is
Scrobicularia plana in radials: 1 to 4, Hediste
diversicolor in radials: 1 to 6 and Hydrobia ulvae in
radials: 0 to 8;
Recovery of an Estuarine Ecosystem after the Stopping of Wastewater Discharges: Macrobenthic Community Characterization in the
Estuary of Oued Souss (Southwestern Morocco)
333
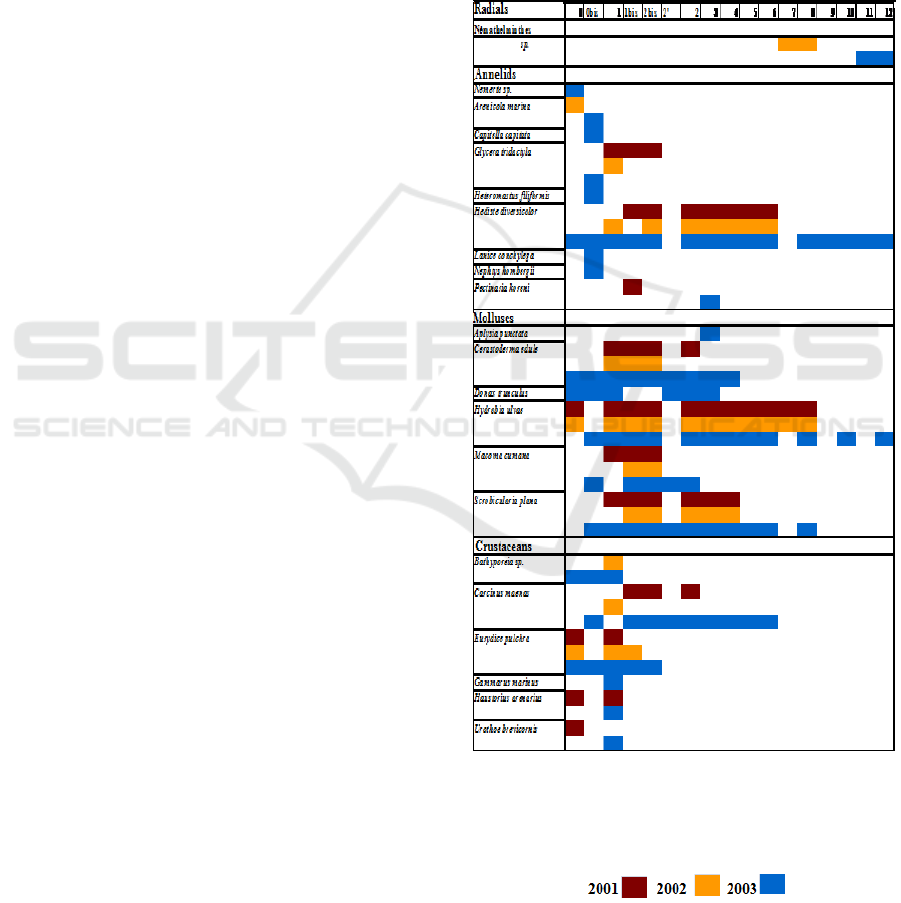
- a fourth group, spotted in 2002 alone, and
represented by the parasitic nematode which is
confined upstream of the estuary, at the level of
radials 7 and 8.
Regarding the stations beyond radial 8, they
constitute an azo-zone.
The study of the coenotic affinity between settlements
of different radials allowed us to separate 2-3 groups
of radials (Figure 3). Thus, in 2001, the dendrogram
distinguishes (Figure 3A): a first group consisting of
radials 0 and 1, 1bis, 2 and 2bis; and a second group
which associates radials 3, 4, 5, 6, 7, and 8. The latter
has two subgroups (3-6 and 7-8). Whereas in 2002,
Figure 3B shows three groups: Group 1 (radials 0, 1,
1bis and 2bis), Group 2 (radials 2, 3, 4, 5 and 6) and
Group 3 (radials 7 and 8).
3.2.2. The Ecosystem after Stopping
Wastewater Discharges
The species composition and distribution, after
discharges stopped, showed a change that was
manifested by a species enrichment, a longitudinal
widening of their distribution area and the
repopulation of radials that were azo during the
discharge period. Four groups are dentified:
- Group 1. (Radials, close to the sea: 0, 0bis, 1). It
is composed of: Arenicola marina, Capitella
capitata, Glycera tridactyla, Heteromastus filiformis,
Lanice conchylega, Nephthys hombergii,
Bathyporeia sp., Gammarus marinus, Haustorius
arenarius, Urothe brevicornis, and finally Eurydice
pulchra which populated also radials 1 bis and 2 bis;
- Group 2. It is constituted by Cerastoderma
edule, Macoma Cumana and Donax trunculus. This
group has a widening of the distribution range of the
e species that compose it, since we note a penetration
of C. edule in the estuary (station 4), as well as M.
cumana which populates radial 2;
- Group 3. It is composed of species that populate
the most radials. This is the case of S. plana (0 bis to
8), H. diversicolor and H. ulvae (radial 0 or 0 bis to
12). To these species, C. maenas (0 bis to 6) can be
included. Species in this group have all expanded
their range in the estuary.
- Group 4. It consists mainly of nematodes having
migrated upstream of the estuary (radials 11 and 12).
In addition, some species are found exclusively in
radial 3: Pectinaria koreni and Aplysia punctata.
In terms of affinity between settlements (Fig. 3C),
we can classify radials into three groups, after
stopping discharges in the estuary:
- Group 1, consisting of settlements from radials:
0, 0bis, 1, 1bis, 2bis;
- Group 2, counting of settlements from radials: 2,
2 ', 3, 4, and 5;
- Group 3, associating the settlements of radials 6,
8 and those of radials which were azo during
discharges: 9, 10, 11, 12.
Figure 2. Longitudinal distribution of intertidal
benthic macrofauna in the mouth of Oued Souss,
Agadir Bay: during the discharge period (2001 and
2002) and after that period (2003).
ICCSRE 2018 - International Conference of Computer Science and Renewable Energies
334
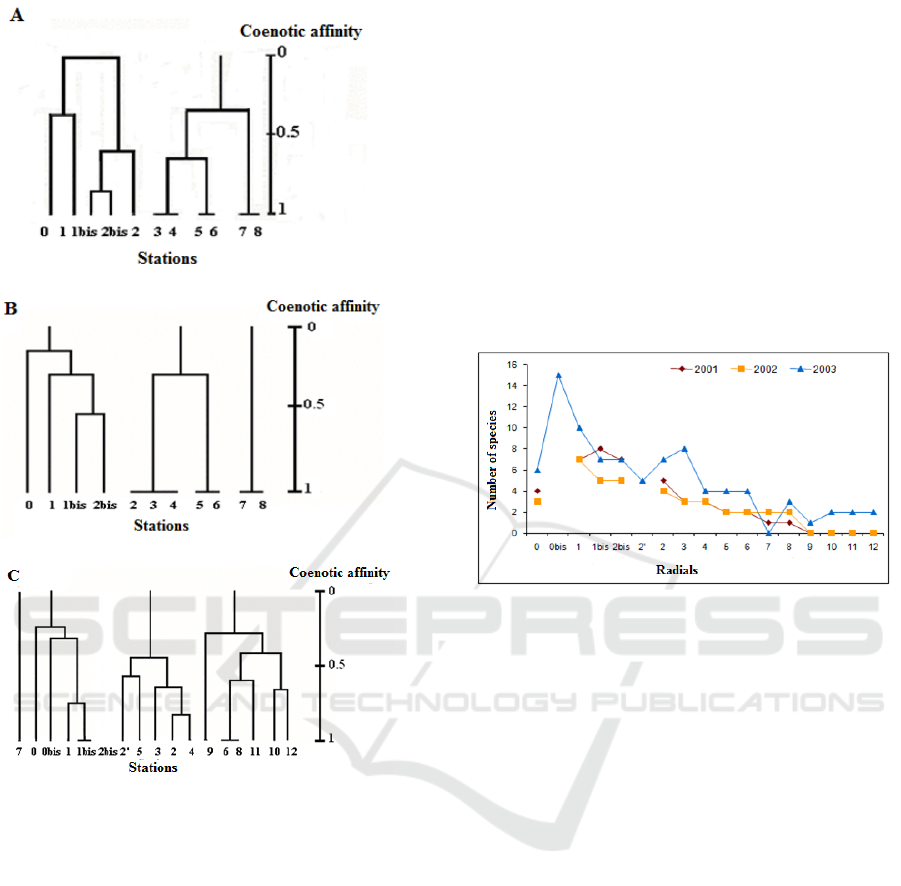
Figure 3. Hierarchical ascending classification of
intertidal invertebrate settlements living during the
period of wastewater discharges (A: 2001, B: 2002),
and after (C: 2003) at the Oued estuary Souss (Bay of
Agadir)
3. 3. Species Richness and Biomass,
before and after the End of
Wastewater Discharge
3. 3.1. Species Richness
During the period when the estuary received
wastewater discharge, the number of species recorded
during the harvest was 14. Figure 4 shows the specific
richness for each radial. There is a negative
correlation between this parameter and the distance of
the radial with respect to the sea. After the cessation
of rejections, the number of species harvested is 22.
Thus, one records an enrichment on almost all the
radials of the estuary with the exception of radial 7,
which has become azoic, after the cessation of
discharges. This last observation is surely in relation
with the change of the river at this level of the estuary.
There is a negative correlation between this
parameter and the distance of the radial with respect
to the sea. After the cessation of rejections, the
number of species harvested is 22.
Thus, one records an enrichment on almost all the
radials of the estuary with the exception of radial 7,
which has become azoic, after the cessation of
discharges. This last observation is surely in relation
with the change of the river at this level of the estuary
Figure 4: Variation of the species richness of the
macrobenthos in the Oued Souss estuary by radial,
before (2001 and 2002) and after (2003) the end of
wastewater discharge
3. 3.2. Biomass
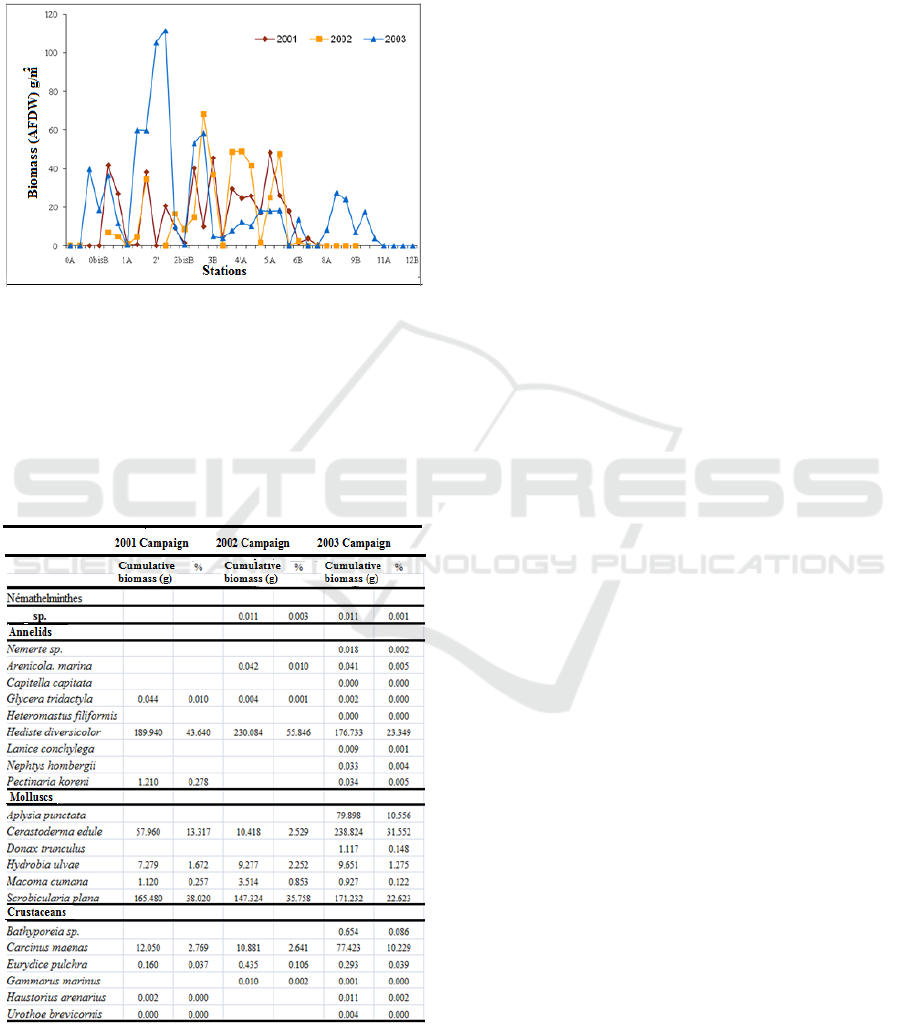
Figure 5 shows differences between the results
obtained for the biomass of fauna recorded by radial
during campaigns carried out before and after the
cessation of discharges.
The results show an increase in the biomass at
radials 0 to 2 of the mouth after the cessation of the
pollution, whereas for radials 3 to 6, there is a
decrease in the biomass.
However, the average biomass calculated after
cessation of discharges has increased significantly. It
is 20.46 g / m
2
(in 2003) compared to 15.54 g / m
2
or
15.84 g / m
2
(in 2001 or 2002 respectively) in the
presence of wastewater discharges
Biomass results by species (all radials combined)
also make it possible to distinguish differences
between the two periods (Table 4).
Thus, during the release period, H. diversicolor has
the largest biomass (43.64 to 55.84%), followed by S.
plana (38 to 35.75%), C. edule (13.32% to 2.53%),
and finally Hydrobia ulvae (1.67 to 2.25%). The
values quoted correspond respectively to the results
Recovery of an Estuarine Ecosystem after the Stopping of Wastewater Discharges: Macrobenthic Community Characterization in the
Estuary of Oued Souss (Southwestern Morocco)
335
of the 2001 and 2002 surveys. Whereas after the
rejection of the discharges, the dominant species in
biomass are in descending order: C. edule which
represents 31.55% of the total biomass, followed by
H. diversicolor (23.35%), S. plana (22.6%), Aplysia
punctata (10.55%) and then C. maenas (10.23%)
Figure 5. Variation of the total biomass of
macrobenthos in the Oued Souss estuary by station
[AFDW in (g/m2)] during wastewater discharges
(2001, 2002) and after (2003).
Table 4. Cumulative biomass by species [AFDW in
(g)] of the intertidal macrobenthos of the Oued Souss
estuary, during the period of wastewater discharges
(2001-2002) and after (2003), all stations being
considered.
4. DISCUSSION
The campaigns that took place while the Oued Souss
estuary was receiving wastewater (2001-2002) made
it possible to draw up the first inventory of the
intertidal zoobenthic fauna in this ecosystem and to
know the structure of this settlement. Regarding
faunal composition, we observe a year-to-year
variation in the total abundance of the individuals
surveyed, without affecting the order in which species
are ranked in relation to their abundance dominance.
In descending order Hydrobia ulvae, followed by
Hediste diversicolor then Scrobicularia plana.
The horizontal distribution shows a succession of
three communities with regards to the coenotic
affinity between the settlements. The first community
occupies radials 0-2 bis (2001), or 0-2 (2002) while
the second includes radials which constitute the
central part of the estuary: 3, 4, 5, 6, then the last
community consists of the species from radials 7 and
8, which are close to the discharges. This latter
community has sometimes a certain affinity with the
community of previous radials (2001) and sometimes
it dissociates completely (2002). In a parallel, study
in which the granulometry and physico-chemistry of
water in the estuary were treated (Gillet et al., 2003).
It has been argued that in the absence of a true hyaline
gradient at the level of the estuary of the Oued Souss,
grain size and the proximity of wastewater discharges
seem to be the factors that most influence the
distribution of species. We have, indeed,
distinguished a first group composed by the radials of
the mouth where the substrate is sandy (coarse sand
to fine for radial 0 to 1a, or sands silted for 2a and 2);
a second group corresponding to the radials where the
sediment becomes much muddier and clayey (radial:
2 or 3 to 6). Finally, a third group of radials 7 and 8,
which corresponds to the zone where the influence of
wastewater discharges is important and where the
surface of the mud flat is very small.
After the cessation of discharges, the total
abundance of the fauna is much greater, the
abundance dominance has undergone modifications
since it is H. diversicolor which becomes the most
abundant, followed by H. ulvae, Cerastoderma edule
then S. plana.
Differences are also observed at the level of the
settlement structure if we continue referring to the
coenotic affinity. Indeed, this parameter allows us to
distinguish three communities: a group at the mouth
(radial: 0 to 2bis), a second group in the central part
of the estuary (radial: 2 'to 5) and a third group which
associates radials upstream (6 and 8) to those which
were azo during discharges (radial: 9 to 12). Radial 7
ICCSRE 2018 - International Conference of Computer Science and Renewable Energies
336

is discarded because at high tide, the seawater does
not reach it any more. This change in the course of
water is surely due to the cessation of discharges.
The preexisting species have seen their range
extend at the level of the estuary. This enlargement is
due either to a colonization of these stations by young
specimens of H. diversicolor, from H. ulvae, S. plana,
or C. edule, or to a migration of these species to these
new territories from nearby habitats. Indeed, several
authors (Mettam, 1981; Lewis et al., 2001; Meziane
& Retière; 2001) report that the populations of H.
diversicolor have a great ability to migrate at any
point in their life cycle. With the exception of
Macoma balthica, which is known for its frequent
migrations (Perkins, 1974), molluscs are not very
mobile. Three species call for particular comments:
- H. ulvae is famous not only for the high densities
it reaches, but also for its controversial behavior.
Authors indicate for this species a tidal cycle of
burial, feeding in a pelagic medium (by flotation) then
after falling on the ground, crawling (Newell, 1962;
Anderson, 1971). On the other hand, other authors
think that buoyancy is accidental, and that burial is a
reaction against dehydration or predators (Barnes,
1981). The dispersion of this small gastropod would
then be due only to the planktonic larval stage or
passive transports, by the movements of the water
(rotation of the adults on the substrate). One can thus
think, after the cessation of the discharges, about a
tidal (floating), exploring the upstream stations. Be
that as it may, the life span of this species is rarely
more than a year and a half. It is reported in the
literature that eggs encapsulated by 3 to 5 are
observable from March to December and larval
arrival occurs three weeks later (Muus, 1986). The
expansion of the range of the species could also be
explained by a new recruitment.
- S. plana, meanwhile, can make rare
displacements in the horizontal direction (Hugues,
1970). Six months after the cessation of the
discharges, the longitudinal widening of the range of
the scrobiculars upstream is surely due to the
installation of juveniles especially as this species
presents, at the level of Oued Souss, two recruitment
periods, from February to March and from late spring
to early autumn (Bergayou & Moukrim, 2005).
- C. edule also has a longitudinal widening of its
distribution on the longitudinal plane. As this species
is living and growing in the sand, the cessation of the
flow of wastewater in the estuary and the absence of
another flow of fresh water in the estuary make the
estuary more susceptible to marine hydrodynamics,
hence the deposit of a thin layer of sand and the
installation of hulls a little further upstream (station
4). This hypothesis is based on the findings of a study
at the level of the Canche estuary (northern France)
(Dobroniak, 2000). The author explains that the
settlements have a certain stability over time, but
migrations could be caused by the morpho-
sedimentary upheavals caused, among other things,
by the hydro dynamism and the geographical
modifications of the bed of the Canche, the
distribution of the biocenoses being essentially due to
these physical factors (Dobroniak, 2000). Another
argument is that the hull is fairly sensitive to
pollution, whatever its nature, according to a study in
the Kinneil mudflats at the Forth estuary in Scotland
(Mac Lusky, 1981). In these mud flats, which receive
domestic, chemical or oil effluents, C. edule does not
live in the sediment near the source of pollution, but
is found 1.5 km from the effluents; and its maximum
abundance is between 1.5 and 2.2 km. In the present
study on Oued Souss, this bivalve was found about
two kilometers from the source of pollution during
discharges and its presence in 2003 in stations 3 and
4 is surely a consequence of the cessation of
pollution.
In marine habitats, the phenomenon of restocking
may depend on the free surfaces in question. For
small areas, restocking is rapid and is done through a
large variety of pioneer opportunistic species (Frid,
1989). For large areas, this repopulation can take
months or years (Beukema et al., 1999). However, in
a more recent study of the Cochin estuary of the
tropical monsoon (India) (Rehitha et al., 2017) where
sites that were or were not subject to dredging were
compared, the authors highlighted the dominance of
a single opportunistic benthic taxon that has settled in
sites that have been dredged.
In this study, only the most downstream, marine-
influenced stations experienced enrichment of new
species. Upstream, preexisting species have
expanded their range. Similar results have been
reported on mudflats at Dutch Wadden Sea (Holland)
(Beukema et al., 1999) and at Clonakily Bay, West
Cork, Ireland (Lewis et al., 2001).
4.1 Species Richness
Specific wealth was very low during the discharge
period (14 species). Indeed, the number of species
usually encountered in an estuary varies between 30
and 300 species. By way of comparison, authors have
identified 32 species in the Loire estuary (Marchand,
1972; Robineau, 1986), 66 species in the Gironde
estuary (Bachelet et al., 1981), 86 species in the
Tagus estuary (Portugal) (Calvario, 1984), 264
species in the Bou Regreg estuary (Morocco)
Recovery of an Estuarine Ecosystem after the Stopping of Wastewater Discharges: Macrobenthic Community Characterization in the
Estuary of Oued Souss (Southwestern Morocco)
337

(Elkaim, 1974; Elkaim, 1976); or 52 species in the
same estuary (Cheggour, 1988) after the Sidi
Mohamed Ben Abdellah dam was commissioned in
1974 in the Bou Regreg basin and, in more recent
studies, 37 species for the macrobenthos furniture in
the Smir lagoon in northern Morocco (Chaouti &
Bayed, 2005) and 57 taxa at the Khnifiss lagoon in
southern Morocco (Lefrere et al., 2015). In the case
of the estuary of Oued Souss, the factors explaining
this faunistic poverty are numerous: the freshwater
inputs are very low because of the rare rainfall; this
phenomenon is further accentuated by the installation
of dams on the bed of the Oued, the most important
of which is that of Aoulouz, 150 km upstream; in
addition, the pollutant load is important because of
urban discharges. Moreover, the lesser diversity of
habitats (lack of rocky substrates, absence of sea grass
beds) at the level of the estuary and its geographical
position with a certain number of species which are at
the southern limit of their distribution area explain to
some extent this low specific wealth.
After the discharges, we counted 22 taxa. This
observation puts the index on the negative effect of
wastewater. However, this is a punctual result and
should be taken with caution. Indeed, the specific
wealth, which reflects stability, depends first and
foremost on breaks in the climax of the ecosystem:
maximum in average conditions, it decreases when
disturbances are important and / or frequent (Connell,
1978). Secondly, the biological regulation of
communities can only take place when the resources
of the environment are fully utilized. Thus, when the
environment remains stable for a very long time,
resources become restricted and competition sets the
limits of the number of species. The dynamic
equilibrium is then reached (Huston, 1994; Pickett &
Cadenasso, 1995): the composition of the settlement
varies according to the colonization and results in a
stable global structure determined by the physical
environment and the predation.
4.2 Biomass
The average biomass of the harvested fauna has
varied. Thus, during the discharge period, it was
15.54 g/m² then 15.84 g/m² (in 2001 and 2002
respectively), with three species (H. ulvae, H.
diversicolor and S. plana) which constitute the
majority: 83.33 to 93.85% respectively for the first
two campaigns. These species are typical of Atlantic
estuaries and are known for their tolerance (Gonzales
Oreja & Sais Salinas, 2003).
On the other hand, after the cessation of
discharges, the average biomass was 20.56 g / m², of
which 78.79% is represented by the four dominant
species in abundance: H. diversicolor, S. plana, C.
edule and H. ulvae. In order of dominance in biomass,
the cockles come first with 31.52% of the total
biomass. This is important and can be explained by
the spectacular expansion in terms of numbers
marked by the species.
The other three species represent only 47.25% of
the total biomass. If we consider the two species S.
plana or H. diversicolor, their total abundance marks,
respectively for the two species, an increase of 1.5 to
3.4 times and 1.89 to 4.13 compared to the period of
discharges. This increase is not accompanied by an
increase in the biomass of these two species.
Knowing their diets: mixed for S. plana (detritivore
at low tide and suspensivore at high tide) and
detitivore for H. diversicolor, we can think of a
slimming of individuals in relation to the decline in
the rate of organic matter noted in the sediment after
the cessation of discharges (Ait Alla et al., 2006b).
In a comparative study of H. diversicolor,
populations of the Bou Regreg estuary (Morocco)
(Gillet, 1986) with populations of this species from
the Ythan (Scotland) estuary (Chambers & Milne,
1975), the author (Gillet, 1986) reaches the same
findings by emphasizing that for neighboring
densities. The highest biomasses corresponded to the
populations of the stations close to the sewage outlets,
and thus whose sediment contained a high level of
organic matter.
Still regarding the biomass, our results are
comparable to those obtained in the Gironde estuary
(France) where 90% of the average biomass
(estimated at 10.4 g / m²) consists of the biomasses of
three dominant species (Bachelet et al., 1981). In
comparison, in a study on mud flats at Duch Wadden
Sea (Holland), an average biomass of 26.6 g/m²
(Beukema, 1976) and a biomass of 13.2 g/m² in the
Lynher estuary (England) are reported (Warwick &
Price, 1975). However, it should be noted that this
type of comparison is difficult because the methods
used are not always homogeneous and the
environmental conditions are rarely taken into
account.
5 CONCLUSIONS
This study proposes an inventory of the intertidal
benthic system of the Oued Souss estuary from a
structural point of view. First of all, it allowed us to
acquire a qualitative (species list, specific wealth) and
quantitative (abundance, biomass, species density)
database of intertidal benthic macrofauna. It
represents a reference state. This tool was used to
ICCSRE 2018 - International Conference of Computer Science and Renewable Energies
338

respond to requests for information about the quality
of the environment immediately after the cessation of
wastewater discharges and could serve as a basis for
conducting impact studies later.
However, while the spatial dimension is relatively
well documented, the temporal dimension was not
taken into account in this study. Finally, we suggest a
continuation of the investigations along the estuary
according to a monthly or seasonal monitoring in
order to be able to establish the structure of the
benthic population over time (seasonal and
interannual variations) and to see the new state of
equilibrium, which must be currently reached by the
different zoobenthic populations of the estuary.
REFERENCES
Agnaou, M., Ait Alla, A., Ouassas, M., Bazzi, Lh., El
Alami, Z., Moukrim, A., 2014. Assessment of
organochlorine pesticides contamination of Oued Souss
estuary (South of Morocco): Seasonal variability in
sediment and a detritivore annelid Neries diversicolor.
J. Mater. Environ. Sci. 5 (2) 581-586 ISSN : 2028-2508
CODEN : JMESCN 581.
Ait Alla A., Mouneyrac C., Durou C., Moukrim A., Pellerin
J., 2006a. Tolerance and biomarkers as useful tools for
assessing environmental quality in the Oued Souss
estuary (Bay of Agadir, Morocco). Comp Biochem
Physiol C Toxicol Pharmacol. 143, (1): 23-29.
Ait Alla, A., Gillet, P., Deutsch, B., Moukrim, A.,
Bergayou, H., 2006b. Population dynamics and
secondary production of Hediste Diversicolor (O. F.
Müller, 1776), (Polychaeta, Nereidae) in estuary of the
Oued Souss, Bay of Agadir, Morocco. Estuarine,
Coastal and Shelf Science, 70: 633-642.
Anajjar, E.M., Chiffoleau, J.F., Bergayou, H., Moukrim,
A., Burgeot, T., Cheggour, M., 2008. Monitoring of
Trace Metal Contamination in the Souss Estuary (South
Morocco) Using the Clams Cerastoderma edule and
Scrobicularia plana. Bull. Environ. Contam. Toxicol.
80:283–288.
Anderson, A. 1971. Intertidal activity, breeding and the
floating habit of Hydrobia ulvae in the Ythan estuary.
J. Mar. Biol. Ass. U. K.., (51) :423-437.
Bachelet, G., Bouchet, J.M., Lissalde, J.P., 1981. Les
peuplements benthiques de la Gironde : biomasse,
productivité et évolution structurale. Oceanis, 6, (6):
593-620.
Barnes R.S.K., 1981. Behavioural activities and ecological
strategies in the intertidal gastropod Hydrobia ulvae in
Feeding and survival strategies of estuarine organisms.
N.V. Jones, W.F. Wolff Eds., Plenum Press. New York
& London, 79-90.
Bergayou, H & Moukrim, A., 2005. Cerastoderma edule
(Linné, 1758) et Scrobicularia plana (da Costa, 1778):
étude comparative de la croissance des mollusques et
des générations annuelles dans l’estuaire de l’Oued
Souss (sud-ouest du Maroc) sous climat aride. Haliotis,
(34) : 49-58.
Bergayou, H., Moukrim, A., Mathieu, M., Gimazane, J.P.,
2008. Reproduction of the cockle Cerastoderma edule
(Linné, 1758) in the estuary of Oued Souss
(southwestern Morocco). Ibérus, 26 (1): 29-42.
Bergayou, H., Mouneyrac, C., Pellerin, J. Moukrim, A.,
2009. Oxidative stress responses in bivalves
(Scrobicularia plana, Cerastoderma edule) from the
Oued Souss estuary (Morocco). Ecotoxicol. Environ.
Safety, 72 (3): 765-769.
Beukema, J.J., 1976. Biomass and species richness of the
macrobenthic animals living on the tidal flats of the
Dutch Wadden Sea. Neth. J. Sea Res (10): 236-261.
Beukema, J.J., Flach, E.C., Dekker, R., Starink, M., 1999.
A long-term study of the recovery of the
macrozoobenthos on large defaunated plots on a tidal
plot in the Wadden Sea. J. Sea. Res. (42): 235-254.
Calvario, J., 1984. Etude préliminaire des peuplements
benthiques intertidaux (substrats meubles) de l’estuaire
du Tage (Portugal) et sa cartographie. Publ. Museum.e
laboratorio Zoologico Antropologico., (11) : 187-205.
Chambers, M.R. & Milne, H. 1975. Life cycle and
production of Nereis diversicolor O. F. Müller in the
Ythan Estuary Scotland. Estu. Cost. Mar. Sci., (3): 133-
144.
Chaouti, A. & Bayed, A., 2005. Diversité taxonomique et
structure de la macrofaune benthique des substrats
meubles de la lagune de Smir (nord-ouest du Maroc)
Travaux de l'Institut Scientifique, Rabat, série générale.
A. Bayed & F. Scapini (éditeurs), n°4, 33-42 33.
Cheggour, M., 1988. Contribution à l’étude d’un milieu
paralique : l’estuaire du Bou Regreg (côte atlantique
marocaine). Thèse Doct. 3ème cycle. Ecole Normale
Supérieure Takaddoum, Rabat. 327 p.
Connell, J.H., 1978. Diversity in tropical rain forests and
coral reefs. Science, (199): 1302-1310.
Dakki, M., El Agbani, M.A., Qninba, A., Benhhoussa, A.,
1995. Recensement hivernal d’oiseaux d’eau au
Maroc : janvier 1995. Documents de l’Institut
Scientifique, Rabat (18).
Dobroniak, C., 2000. Géomorphologie, Hydrodynamisme,
et écologie tempéré macrotidal : l’Arthie, Manche
Orientale, France. Thèse de doctorat de Géographie
physique, Université du Littoral, Wimereux, 306p.
El Bekkay, M., 2013. Contribution à l’étude du rôle des
aires protégées en tant qu’outil pour la conservation de
la biodiversité et la valorisation des territoires : Cas du
site Ramsar de l’estuaire de l’oued Massa (Parc
National Souss Massa). Thèse nationale, Univ. Ibn
Zohr, Agadir, 190 p.
El Hamidi, F., Banaoui, A., Azdi, M., Kaaya, A., Zekhnini,
A., Moukrim, A., 2002. Utilisation de la réponse de
quatre biomarqueurs d'exposition chez les bivalves
Perna perna et Donax trunculus pour l'évaluation de la
pollution dans la baie d'Agadir (sud du Maroc).
Haliotis, 32, 21-32.
Elkaim, B., 1974. Contribution à l’étude écologique d’un
estuaire atlantique marocain : l’estuaire du Bou Regreg.
Thèse Doct. Ès-Sci., Bordeaux I, 251 p.
Recovery of an Estuarine Ecosystem after the Stopping of Wastewater Discharges: Macrobenthic Community Characterization in the
Estuary of Oued Souss (Southwestern Morocco)
339

Elkaim, B., 1976. Bionomie et Ecologie des peuplements
des substrats meubles d’un estuaire atlantique
marocain : l’estuaire du Bou Regreg. II. Unités
indicatrices peu liées à l’étagement ou médiolittorales.
Vie Milieu, Vol. XXVI, fasc. 2, série B : 199-241.
Elliot, J.M. & Descamps, H., 1973. Guide pour l’analyse
statistique des échantillons d’invertébrés benthiques.
Ann. Limn., (2): 79-120.
Frid, C.L.J., 1989. The role of recolonization in benthic
communities, with special reference to the
interpretation of predato-induced effects. J. Exp. Mar.
Biol. Ecol. (126) : 163-171.
Gillet, P., 1986. Contribution à l’étude écologique des
Annélides Polychètes de l’estuaire de Bou Regreg
(Maroc). Thèse de Doctorat, Université d’Aix-
Marseille, 215 p.
Gillet, P., Gorman, E., Tallec, P., Moukrim, A., Mouloud,
M., Ait Alla, A., Bergayou, H., & Kaaya A., 2003.
Impacts des rejets urbains sur les communautés
benthiques intertidales de l’embouchure de l’Oued
Souss, baie d’Agadir, Maroc. J. Rech. Océanogr. (28) :
39-44.
Gonzalez Oreja, J.A. & Sais Salinas, J.I. 2003. Recovery
simulations of grossly polluted sediments in the Bilbao
Estuary. Mar. Poll. Bull., (46): 42-48.
Hugues, R.N., 1970. Population dynamics of the bivalve
Scrobicularia plana (Da Costa) on an intertidal mud-
flat in North Wales. J. Anim. Ecol., (39) : 333-356.
Huston, M. A., 1994. Biological Diversity: The
Coexistence of Species on Changing Landscapes.
Cambridge University Press, 708 pp.
Idardare, Z., 2005. Utilisation des biomarqueurs pour
l’évaluation de l’état de santé de deux écosystèmes
côtiers : cas de l’estuaire de l’Oued Souss et de la plage
de Bouadisse. Mémoire de DESA, université Ibn Zohr,
Agadir, 40 p.
Id-Halla, M., Kaaya, A., Touyer, O., Texier, H., Moukrim,
A., 1998. Etude de la physico-chimie des eaux usées
des trois principaux rejets du Grand Agadir et de leur
impact sur la qualité de l'eau de mer. Journal Sciences
de l’eau.
Lance, G.N. & Williams, W.T., 1967. A general theory of
classification sorting strategies. I. Hierarchical System
Computing Journal, (9) : 373−380.
Lefrere, L., 2005. Contribution au suivi du rétablissement
de l’estuaire de l’Oued Souss après l’arrêt du rejet des
eaux usées du grand Agadir. Mémoire de DESA,
université Ibn Zohr, Agadir, 33 p.
Lefrere, L., Ouassas, M., Guillois, B., Gillet, P., Moukrim,
A. 2015. Macrobenthic community structure of soft-
bottom sediments in the Khnifiss lagoon, South of
Morocco. J. Mater. Environ. Sci. 6 (11) 2226-2236
Lefrere et al. ISSN : 2028-2508 CODEN : JMESCN
2226
Legendre, P. & Legendre, L., 1998. Numerical Ecology,
2nd English edition. Developments in environmental
modelling, Elsevier Science BV, Ed., Amsterdam. 853
p.
Lewis, L.J., Davenport, J., Kelly, T.C., 2001. A study of the
impact of a pipeline construction on estuarine benthic
invertebrate communities. Est. Coast. Shelf. Sci., (55):
213-221.
Mac Lusky, D.S., 1981. The estuarine ecosystem. Blackie
ed., Glasgow & London, 150 p.
Marchand, J., 1972. Bionomiebenthique de l’estuaire de la
Loire. Rev. Trav. Inst. Pêches mar. 36 (1): 47-67.
Mettam, C., 1981. Survival strategies in estuarine Nereids.
In: Feeding and survival strategies of estuarine
organisms, Jones N.V.& Wolff W.J., eds. Plenum
Press, London, 65-77.
Meziane, T. & Retière, C., 2001. Role of biotic interactions
seasonal migrations of the macrozoobenthos living in
the upper tidal-flat of the Mont-Saint-Michel bay,
France. Oceanologica acta. 24, (6): 1-7.
Moukrim, A., Chiffoleau, J.F., Cheggour, M., Burgeot, T.,
2008. Changes in the sediment trace metal
contamination after the commissioning of a municipal
wastewater treatment plant in the Souss estuary (South
Morocco). Bulletin of Environmental Contamination
and Toxicology (0007-4861) (Springer), 2008-06, Vol.
80, N. 6, P. 549-554.
Muus B.J., 1986. The fauna of Danish estuaries and
lagoons. Distribution and ecology of dominating
species in the shallow reaches of the mesohaline zone.
Medd. fra. Danm. Fish. Og Havunders, N. S. 5 (1): 3-
316.
Newell, R.C., 1962. Behavioural aspects of the ecology of
Perengia (Hydrobia) ulvae. Proc. Zool. Soc. Lond.,
(138) :49-75.
Oubrou, W. & El Bekkay, M., 2014. Rapport sur la
reproduction de l’Ibis chauve dans la région de Sous-
Massa. Haut-Commissariat aux Eaux et Forêts et à la
Lutte Contre la Désertification. Direction Régionale des
Eaux et Forêts et de Lutte Contre la Désertification du
Sud-Ouest.
Perkins, E., 1974. The biology of estuaries and coastal
waters. Academic Press, London, 678 p.
Pickett, S.T.A. & Cadenasso, M.L., 1995. Landscape
Ecology: spatial heterogeneity in ecological systems.
Science, (269) : 331-334.
R. Mimouni, R., Ait Alla, A., Anajjar, E.M., Finance, C.,
Moukrim, A., 2002. Impacts du rejet des eaux usées sur
la qualité microbiologique des plages de la baie
d'Agadir (Maroc). Journal Européen d'Hydrobiologie,
33(1) : 115-123.
Rehitha, T.V., Ullas, Vineetha N G., Benny, P.Y., Madhu,
N.V., Revichandran, C., 2017. Impact of maintenance
dredging on macrobenthic community structure of a
tropical estuary. Ocean & Coastal Management
144, 71-82.
Robineau, B., 1986. Les peuplements benthiques de
l’estuaire de la Loire. Distribution spatio-temporelle.
Reproduction et croissance des Bivalves Tellinidés.
Thèse de Doctorat, laboratoire de biologie marine de
l’Université de Nantes, 298 p.
Snoussi, M., 1988. Nature, estimation et comparaison des
flux de matières issus des bassins versants de l’Adour
(France), du Sebou, de l’Oum-Er-Rbia et du Souss
(Maroc) : Impact du climat sur les apports fluviatiles à
l’océan. Thèse Univ. Bordeaux I: 459p.
ICCSRE 2018 - International Conference of Computer Science and Renewable Energies
340

Warwick, R.M. & Price, R., 1975. Macrofauna production
in an estuarine mudflat. J. Mar. Biol. Ass. U. K., (55) :
1-18.
Recovery of an Estuarine Ecosystem after the Stopping of Wastewater Discharges: Macrobenthic Community Characterization in the
Estuary of Oued Souss (Southwestern Morocco)
341
