
Simulation Study of Thorium Separation from Monazite Mineral
Megawati
1
, Haniif Prasetiawan
1
, Bayu Triwibowo
1
and Anwaruddin Hisyam
2
1
Chemical Engineering Department, Faculty of Engineering, Universitas Negeri Semarang, Semarang, Indonesia
2
Faculty of Chemical and Natural Resources Engineering, Universiti Malaysia Pahang, Pekan, Malaysia
Keywords: Rare Earth, Thorium, Separation, Simulation
Abstract: Growth of electricity demand is rapidly increasing. Several method to supply electricity such as utilizing
renewable energy has been used. Nuclear energy is still the best choice to produce electricity. Instead of
using uranium as a fuel, utilization of thorium as a nuclear power plant fuel has been investigated since
1950. Thorium is much safer than uranium since it does not produce hazardous by product which can be
used as an explosive material. Thorium can be found widely in Indonesia together with uranium and many
other rare earth material such as lanthanides group. Separation is needed before thorium can be used as a
fuel. In this article, preliminary study of thorium separation from monazite mineral has been conducted.
The end product of this study is a plant design for thorium separation process. This study used secondary
data which has been lab scale experimental. The data was then predicted by using mathematical model,
calculated data were then compared to the experimental data. The model used in this study was in
accordance with the experimental data, where R
2
for thorium, uranium and rare earth element are 0.9591,
0.7591 and 0.9889 respectively. The developed model in this study then can be used for thorium separation
process modelling using METSIM.
1 INTRODUCTION
In 2018, the growth of electricity demand in the
world is increasing twice as the global demand of
energy which is 900 TWh. From overall electricity
generation, gas and coal are still have the biggest
contribution. While, the generated electricity from
renewable energy resources only able to supply less
than 10%. More than 90 TWh of electricity
generation contributed by nuclear energy (IEA,
2019). As a promising alternative energy in the
world, there are 450 nuclear reactors in total which
is operated in over 30 countries around the world.
There are six type of different reactor which are used
in the nuclear power plant namely Boiling Light-
Water Cooled and Moderated Reactor (BWR), Fast
Breeder Reactor (FBR), Gas Cooled, Graphite
Moderated Reactor (GCR), Light-Water Cooled,
Graphite Moderated Reactor (LWGR), Pressurized
Heavy-Water Moderated and Cooled Reactor
(PHWR) and Pressurized Light-Water Moderated
and Cooled Reactor (PWR). PWR reactor type is the
most popular in the world which is being used by
more than 60% of nuclear power plant around the
world (PRIS, 2019).
The electricity of nuclear plant was obtained
from spin large turbines which is operated by the
steam from water heating process. The water heating
process is utilizing a heat produced during nuclear
fission process to convert the water into a steam.
uranium oxide (UO
2
) is commonly used as a fuel of
nucler reactor, meanwhile not all of the uranium
product can be used as fuel. There are only 0.7% of
of natural uranium can undergo a fission process and
produce high energy. Naturally, mined uranium
consist of 0.7% uranium-235 (U-235) and 99.2% of
uranium-238 (U-238) (WNA, 2018). In the other
hand, uranium have some disadvantages which are
having deleterious health effect and also create a
radioactivity waste issue. The leaked radiation from
nuclear power plant can last for centuries and create
a problem for the next generations. U-238 also very
dangerous since during the fission process it can
transmute into neptunium and then into plutonium-
239 which is a byproduct that can be used as a
weapon.
Thorium has become an alternative nuclear
power plant fuel to replace U-235. In nature,
thorium is more abundant rather than uranium, Its
oxide form, ThO
2
also relatively inert compared to
Megawati, ., Prasetiawan, H., Triwibowo, B. and Hisyam, A.
Simulation Study of Thorium Separation from Monazite Mineral.
DOI: 10.5220/0009013104550459
In Proceedings of the 7th Engineering International Conference on Education, Concept and Application on Green Technology (EIC 2018), pages 455-459
ISBN: 978-989-758-411-4
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
455
UO
2
which is easily oxidised into a more dangerous
byproduct (Dewita, 2012). Thorium can be found
easily in a mineral monazite which is the second
most important rare earth (RE) mineral source
containing thorium and uranium in associated metals
(Amaral and Morais, 2010). Studies on the thorium
fuel cycle had been started from 1950 and still
continuing until recent centuries (Oettingen and
Skolik, 2016).
There are numerous research have been
conducted to separate thorium from mineral
monazite/monazite sands. Hughes and Singh (1980),
introduced solvent extraction process of monazite
sulphate solution by using secondary amine Adogen
283. A maximum thoria concentration of 18 g/l was
obtained by using ammonium carbonate solution as
a stripping agent in the 9-10 pH range. Ali et al.
(2007) develop a process to obtain the thorium from
the hydrous oxide cake from which uranium has
been removed. It was found that Aliquat-336 can be
prefentially extract thorium from HNO
3
solutions.
The extraction efficiency was found to be 80% and
the stripping process efficiency was 82%. Amaral
and Morais (2010) investigation, stated that four
stages of extraction step, five stages of stripping
process and one stage of solvent generation able to
separate 99.9% of thorium and 99.4% of uranium
from aquaeous solution with only less than 0.001
g/L metal contents in the extract phase.
Further process of thorium separation from
uranium has been investigated by Trinopiawan and
Sumiarti (2012). Two different solvent were used to
precipitate the thorium from uranium, it gave a
satisfying results where sulfuric acid able to obtain
96.99% while chloride able to obtain 98.05%.
Simulation study in the research of rare earth
elements especially in nuclear power plant is very
impoertant. It might help the researcher on
predicting the separation process of thorium to
obtain an advance and optimum process. Larochelle
and Kasaini (2016) were presenting an alternative
model for designing and optimizing rare earth
element solvent extraction process using METSIM.
The simulation results has been compared to pilot
plant and it shows a good accordance with the real
solvent extrction process. Advantages of process
simulation in rare earth process are able to minimize
the complexity of the design by only using a simple
batch extraction data and also able to optimize the
process theoretically based on existing variables and
parameters.
This research will discuss on the preliminary of
simulation study of thorium separation process plant.
In the future, this study might have a worth
contribution for the thorium separation process
plant.
2 RESEARCH METHODOLOGY
2.1 Thorium Separation Process Flow
Diagram
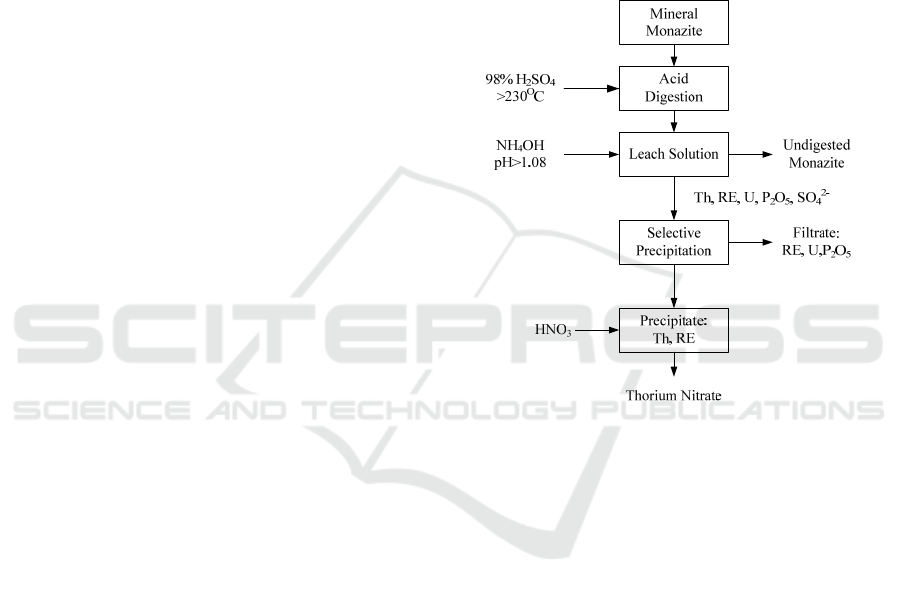
The flow diagram process for thorium separation
process is shown in Figure 1.
Figure 1: Flow diagram process for monazite digestion
and thorium recovery (Al-Areqi, 2016).
2.2 Data Observation
The secondary data was obtained from the previous
work related to thorium separation process. Data set
of monazite digestion process was obtained from
Anggraeni et al. (2015). While data for thorium
separation from uranium by using precipitation
process were obtained from Trinopiawan & Sumiarti
(2012).
2.3 Simulation Study
This simulation study was conducted by using
METSIM software with licensed owned by
Universiti Malaysia Pahang.
EIC 2018 - The 7th Engineering International Conference (EIC), Engineering International Conference on Education, Concept and
Application on Green Technology
456
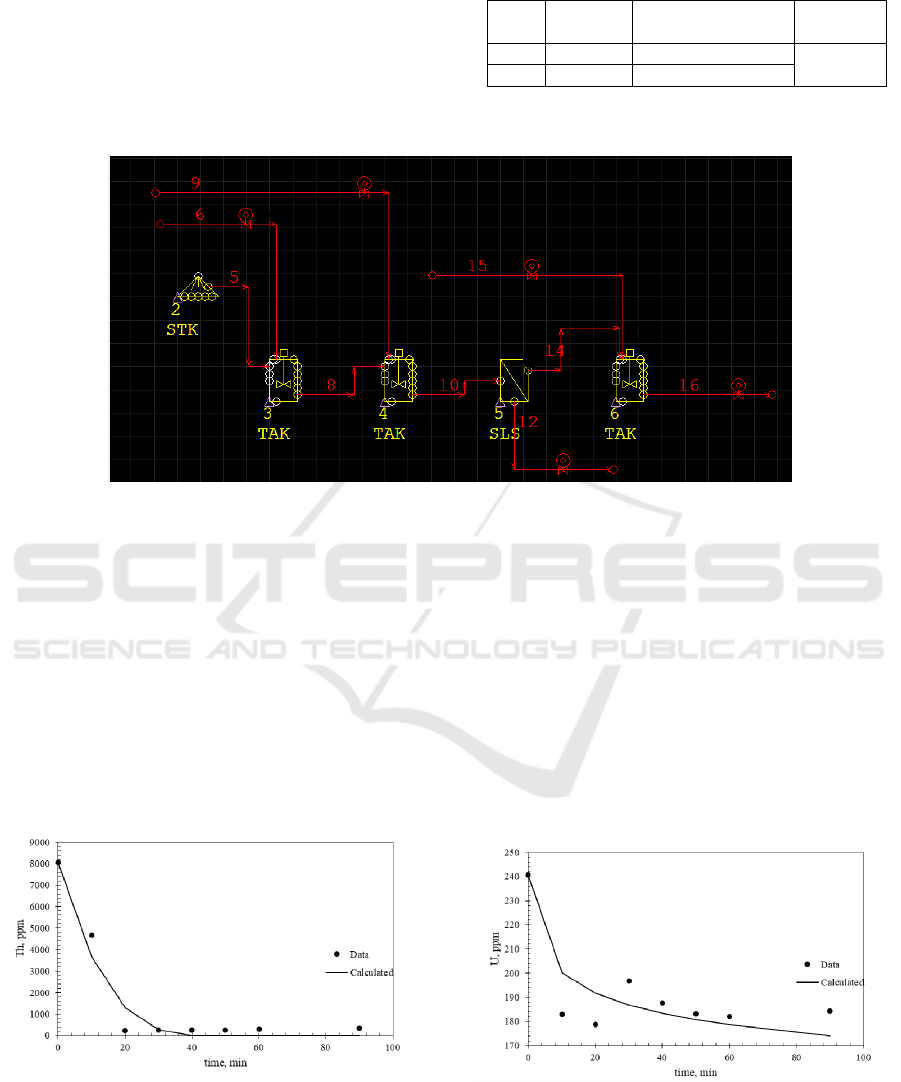
3 RESULTS AND DISCUSSION
Based on Figure 1, the flow sheet of thorium
separation process was created in METSIM
Software. The detail of the flow sheet is shown in
Figure 2.
Table 1: Kinetic constant for thorium concentration data
regression analysis.
Vari
able
Value 95% confidence
interval
R
2
k 0.6933 ± 0.0039124
0.9591
n 1.1009 ± 0.0357526
Figure 1: Flowsheet of Thorium Separation Process.
Each of the simulated equipment, required a specific
input equation to represent the current process. Acid
digestion process of a monazite, requires a kinetic
parameter to simulate the rare earth material
separation from monazite mineral. The data was
obtained from Trinopiawan and Sumiarti (2012).
The predicted model of ihorium in acid digestion are
shown in Figures 2 and Table 1. While the kinetic
model used is shown in Equation 1.
,
1
/
(1)
Figure 2: Simulation result of thorium concentration
over digestion time.
Based on the R
2
value, the simulated result of
Thorium concentration was in accordance with the
data experiment.
In the digestion process of monazite, besides
thorium there is also uranium which already
commonly used in nuclear power plant as a fuel. The
model for uranium separation during digestion
process also predicted using Equation 1 and the
results are shown in Figure 3 and Table 2.
Figure 3: Simulation result of uranium concentration
over digestion time.
Simulation Study of Thorium Separation from Monazite Mineral
457
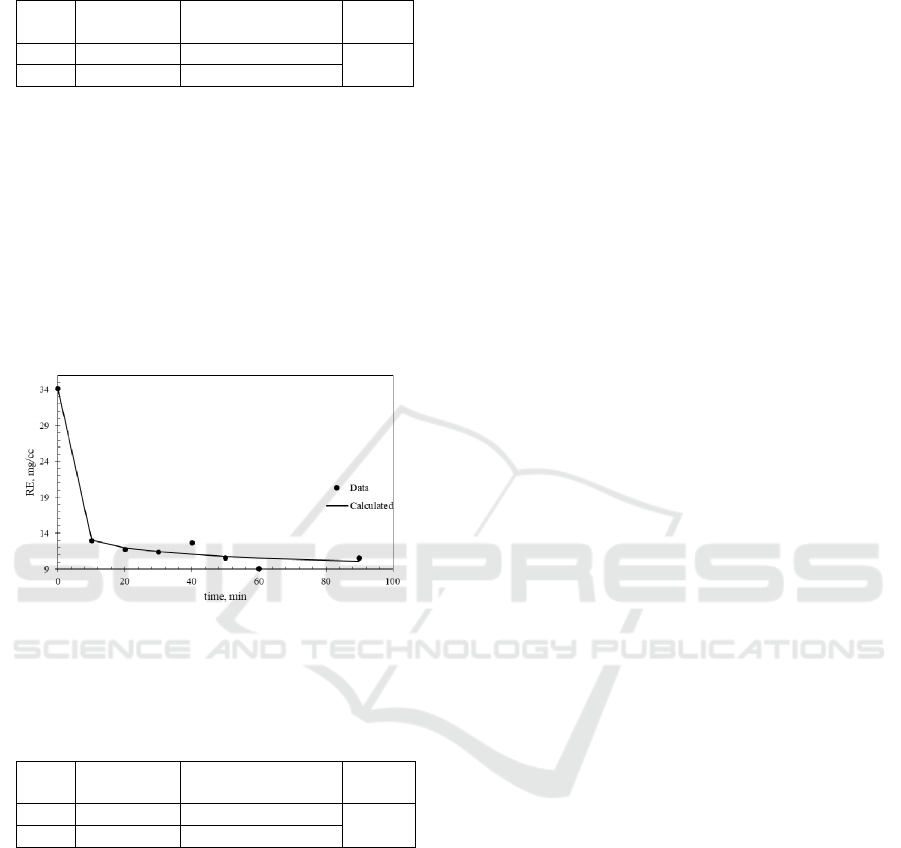
Table 2: Kinetic constant for uranium concentration data
regression analysis.
Vari
able
Value 95% confidence
interval
R
2
k 16.38806 ± 3.583056
0.7591
n 2.38E-38 ± 4.48E-37
A scattering data experiment used in this
simulation caused a slight value of deviation and
affect the R
2
value.
The other components inside monazite mineral
were analyzed as a total Rare Earth Element (REE)
concentration. This component can not be eliminated
during the simulation due to its existence is very
large in monazite mineral. The predicted model of
REE concentration are shown in Figure 4 and Table
3.
Figure 4: Simulation result of rare earth (RE)
concentration over digestion time.
Table 3: Kinetic constant for rare earth (RE) concentration
data regression analysis.
Vari
able
Value 95% confidence
interval
R
2
k 9.57258 ± 7.307524
0.9889
n 3.33E-12 ± 6.21E-11
From Figure 4 and Table 3, it can be seen that
the equation able to represents the rare earth material
digestion process over acid condition. The R
2
value
is close to 1 indicated that the model is fit enough
with the experimental data.
In the future, this model is going to be used in
the METSIM simulation to predict the thorium
separation process from monazite mineral.
4 CONCLUSION
In this study, the flow sheet of thorium separation
from monazite mineral has been prepared. All the
model have been developed by using secondary
data. The result shows that model for thorium and
RE digestion process is in accordance with the
experimental data, while model for uranium
digestion process is quite far from the data but it still
manageable to be used in the upcoming simulation.
ACKNOWLEDGEMENTS
The authors would like to thank the grant project of
DIPA UNNES with reference number
042.01.2.400899/2018 for sponsorship.
REFERENCES
Al-Areqi, W. M., Aniza, C. N., Majid, A. A. & Sarmani,
S., 2016. “Separation and Radiological Impact
Assessment of Thorium In Malaysian Monazite
Processing”, Malaysian Journal of Analytical Science.
Vol. 20, No. 4, pp. 770 – 776.
Ali, A. M.I, Daoud, j. A. & Aly, H. F., 2007. “Recovery of
thorium ( IV ) from leached monazite solutions using
counter-current extraction”, International Journal of
Mineral Processing, Vol. 81, pp. 217 – 223.
Amaral, J & Morais, C., 2010. “Thorium and uranium
extraction from rare earth elements in monazite
sulfuric acid liquor through solvent extraction”,
Minerals Engineering, Vol. 23, No. 6, pp. 498 – 503.
Amaral, J. C. B.S. & Morais, C. A., 2010. “Thorium and
uranium extraction from rare earth elements in
monazite sulfuric acid liquor through solvent
extraction”, Minerals Engineering, Vol. 23, No. 6, pp.
498 – 503.
Anggraini, M., Sarono, B., Waluyo, S., Rusydi, R.,
Sujono, S. 2015. “Pengendapan Uranium dan Thorium
Hasil Pelarutan Slag II”, Vol. 36, No. 2, pp. 125 – 132.
Dewita, E., 2012. “Analisis Potensi Thorium Sebagai
Bahan Bakar Nuklir Alternatif PLTN”, Jurnal
Pengambangan Energi Nuklir, Vol. 14, No. 1, pp. 45 –
56.
Hughes, K. & Singh, R., 1980. “The Isolation of Thorium
from Monazite by Solvent Extraction : Part 1”,
Hydrometallurgy, Vol. 6, pp. 25 - 33
IEA, 2019. Global Energy and CO
2
Status Report: The
Latest Trends in Energy and Emmisions in 2018,
International Energy Agency, France.
Oettingen, M & Skolik, K., 2016. “Numerical design of
the Seed-Blanket Unit for the thorium nuclear fuel
cycle”, E3S Web of Conference, Vol. 10, pp. 00067-1
– 00067-5.
PRIS, 2019. Operational & Long-Term Shutdown
Reactors, https://pris.iaea.org/PRIS/WorldStatistics/
OperationalReactorsByType.aspx, accessed at 31
st
March 2019.
EIC 2018 - The 7th Engineering International Conference (EIC), Engineering International Conference on Education, Concept and
Application on Green Technology
458

Trinopiawan, K. & Sumiarti, S., 2012. “Pemisahan
Thorium Dari Uranium Pada Monasit Dengan Metode
Pengendapan”, Vol. 33, No. 1, pp. 55 – 62.
WNA, 2018. Nuclear Power Reactors, World Nuclear
Association, London, United Kingdom.
Simulation Study of Thorium Separation from Monazite Mineral
459
