
Simulation of the Extractive Distillation using Ethylene Glycol as an
Entrainer in the Bioethanol Dehydration
Dhoni Hartanto
1
, Akhmad Sutrisno
1
, Viona Widya
1
, Asalil Mustain
2
, Prima Astuti Handayani
1
,
Haniif Prasetiawan
1
, Achmad Chafidz
3
and Ianatul Khoiroh
4
1
Department of Chemical Engineering, Faculty of Engineering, Universitas Negeri Semarang,
Kampus Sekaran Gunungpati, Semarang, 50229, Indonesia
2
Department of Chemical Engineering, Politeknik Negeri Malang, Jl. Soekarno Hatta No. 9, Malang 65141, Indonesia
3
Department of Chemical Engineering, Universitas Islam Indonesia, Yogyakarta, 55584, Indonesia
4
Department of Chemical and Environmental Engineering, Faculty of Engineering, University of Nottingham Malaysia
Campus, Jalan Broga, Semenyih, 43500 Selangor Darul Ehsan, Malaysia
prima@mail.unnes.ac.id, haniifprasetiawan@mail.unnes.ac.id, achmad.chafidz@uii.ac.id,
Ianatul.Khoiroh@nottingham.edu.my
Keywords: Bioethanol, Extractive Distillation, Ethylene Glycol, Simulation.
Abstract: In this work, the dehydration of bioethanol via extractive distillation using ethylene glycol as an entrainer
was simulated using Aspen Plus software platform. RadFrac module for distillation was performed
including column for the ethylene glycol recovery which represented the industrial condition. The Non
Random Two Liquids-Hayden-O'Connell (NRTL-HOC) thermodynamic model was used in the simulation.
The results show that the possibility of producing high purity bioethanol through the extractive distillation
using ethylene glycol as an entrainer. The most suitable configuration in extractive distillation column is 23
theoritical stages with the best binary and entrainer feeding stages are 13 and 23, respectively using ethylene
glycol as an entrainer with reflux ratio of 2. The effect of main variables to the extractive distillation were
also obtained.
1 INTRODUCTION
The use of alternatif energy become an important
concern for human kind to reduce the draw back of
the conventional fuel. Biofuels such as bioethanol
and biodiesel have been significantly performed as
subtitutes for fossil fuel energy such as gasoline and
diesel fuel especially in the transportation sector.
Bioethanol is known as a worldwide interest for the
renewable energy because its high energy content
and can be produced from the renewable sources
mainly through the fermentation of sugar . The
production of bioethanol through fermentation
process yielding the purity of 7-12 wt% (Zabed et al.,
2017). Conventional distillation used in the
purification of bioethanol can only produced the
maximum purity of 95.6 wt% due to azeotrope form
of ethanol and water (Dias et al., 2009). On the other
hand, ethanol as a fuel purposes should have the
minimum purifty of 99.5 wt% to meet the product
specification (Zhu et al., 2016).
Several methods were employed to produce high
grade bioethanol such as azeotropic distillation,
adsorption using molecular sieve, pervoration
membranes, and extractive distillation (Xu et al.,
2018) (Frolkova and Raeva, 2010) (Seo et al., 2018)
(Kiss and Suszwalak, 2012). Extractive distillation
has been widely used in the industry as a proven
technology because it has low energy consumption
(Fu, 2004). Feasible entrainer was used in the
extractive distillation to break the azeotrope
(Hartanto et al., 2016). Ethlyne glycol is possible
entrainer proposed to break the azeotrope of ethanol-
water (Kamihama et al., 2012).
Isobaric vapor-liquid equilibria is the basic data
which can be used to optimize the column design
(Hardjono et al., 2017). The vapor-liquid equilibria
can be obtained from experimental and prediction
(Hartanto, Mustain and Nugroho, 2017). In the
450
Hartanto, D., Sutrisno, A., Widya, V., Mustain, A., Handayani, P., Prasetiawan, H., Chafidz, A. and Khoiroh, I.
Simulation of the Extractive Distillation using Ethylene Glycol as an Entrainer in the Bioethanol Dehydration.
DOI: 10.5220/0009013004500454
In Proceedings of the 7th Engineering International Conference on Education, Concept and Application on Green Technology (EIC 2018), pages 450-454
ISBN: 978-989-758-411-4
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
extractive distillation, several research were
conducted to calculate or simulated the extractive
distillation of ethanol dehydration using ethylene
glycol as an entrainer. Black and Ditsler was reported
the comparison of the calculation between the use of
ehtylne glycol and n-pentane in extractive distillation
(Black and Ditsler, 1972). Anisuzzaman et al. also
simulated the extractive distillation using three
solvents of 1,3-butylene glycol, mixture 1,3-butylene
glycol and ethylene glycol, and mixture 1,3-butylene
glycol and glycol ethyl ether using the Aspen
HYSYS Platform (Anisuzzaman et al., 2018). The
mixture of ethylene glycol – calcium chloride was
used in the extractive distillation of ethanol
dehydration simulation using the Aspen Plus
platform (Gil et al., 2008). The experimental and
pilot scale research of extractive distillation using
ethlyne glycol as a solvent were conducted by
Meirelles(Yeh and Berg, 1992). Gil et al. used
glycol-
glycerol as an entrainer in the simulation of ethanol
dehydration using Aspen Plus software platform (Gil,
García and Rodríguez, 2014).
The study of the extractive distillation for the
bioethanol dehydration using ethylene glycol as an
entrainer using the Aspen Plus software platform is not
available in the published literature. In this study, the
effect of stages, reflux ratio (RR), entrainer stage, binary
feed stage to product composition, reboiler and
condensor duty, and energy duty were investigated. The
NRTL-HOC model was used as a thermodynamic
package in the simulation.
2 EXTRACTIVE DISTILLATION
SIMULATION
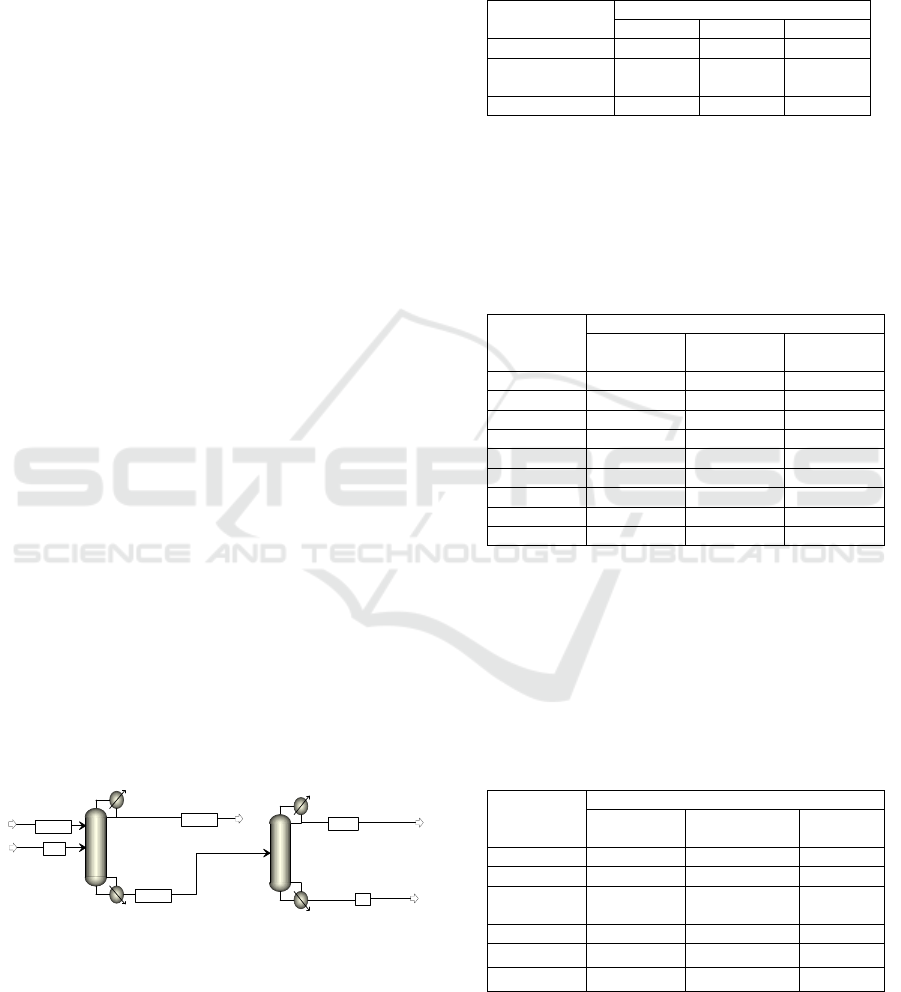
The extractive distilation simulation was presented in
Figure 1 as a process flow diagram which consist of
two columns, one as a extractive distillation column
and one as an entrainer recovery column.
Figure 1: Process flow diagram for the extractive
distillation using ethylene glycol as an entrainer.
The isobaric vapor liquid equilibria of ethanol-
water-ethylene glycol was taken from the reference
(Kamihama et al., 2012). The simulation was
conducted using The NRTL-HOC model as a
thermodynamic package. The binary interaction
parameter of ethanol-water, ethanol-ethylne glycol,
and water--ethylne glycol was taken from Aspen
database which shown in Table 1 with the unit of K.
Table 1: Binary Interaction Parameters*.
Component
Parameters
Aij Aji Cij
ethanol-water 227.56 5196.9 0.4
ethanol-ethylne
glycol
1035.6 708.38 0.23
water--ethylne -2510 2731.3 0.33
*Taken from Aspen Plus physical property databank
The extended Antoine was used in this simulation
to calculate the total pressure and vapor pressure of
the component. The Antoine parameters was listed in
the Table 2 with the unit pressure and temperature
are kPa and K, respectively.
Table 2: Extended Antoine Parameters*.
Parameters
Compound
ethanol water
ethylene
glycol
A
1
74.1675 58.2467 -418.74
A
2
-7827.8 6842.91 7736.16
A
3
0 0 0
A
4
-0.00185 0.00278 -0.0872
A
5
-7.96131 -6.13638 72.7647
10
-15
A
6
0.023 0.0033 0.017
A
7
6 6 6
A
8
302.559 319.267 406.541
A
9
516.2 647.3 645
*Taken from Aspen Plus physical property databank
Some of physical properties were used in the
calculation of phase equilibrium such as critical
temperature (Tc), critical pressure (Pc), critical
volume (Vc), compressibility factor (Zc), dipole
moment (μ), and acentric factor (ω) of each
component. The constant of each physical property
listed in Table 3.
Table 3: Physical Properties Of Pure Component*.
Parameters
Compound
ethanol water
ethylene
glycol
Tc (K) 516.2 647.3 645
Pc (kPa) 6383.48 22048.3 7700.7
Vc
(m
3
/kmol)
0.16673 0.05589 0.18802
Zc 0.248 0.229 0.27
μ (debye)
1.7 1.8 2.2
ω
0.635 0.344 1.17921
*Taken from Aspen Plus physical property databank
The simulation were conducted with 50
theoritical stages in the extractive distillation column.
The mol fraction of entrainer used for all simulation
EXTR-COL
REC-COLM
FEED
SOLVENT
ETHANOL
RICH-SOL
WATER
EG
Simulation of the Extractive Distillation using Ethylene Glycol as an Entrainer in the Bioethanol Dehydration
451
is fixed at 0.059 according to the optimum amount of
entrainer which added into the system to break the
azeotrope of ethanol-water (Kamihama et al., 2012).
The initial input data of the simulation are listed in
the Table 4.
Table 4: Process Design Parameters.
Parameters Value
Feed flowrate (kmol/h) 94.1
Distillate mole flow (kmol/h) 75.28
Ethanol feed mole fraction 0.8
Theoritical stage numbers 50
Entrainer mole fraction 0.059
Feed temperature (
o
C) 25
Entrainer temperature (
o
C) 25
Binary feed stage 10
Entrainer feed stage 5
Pressure (kPa) 101.3
3 RESULTS AND DISCUSSION
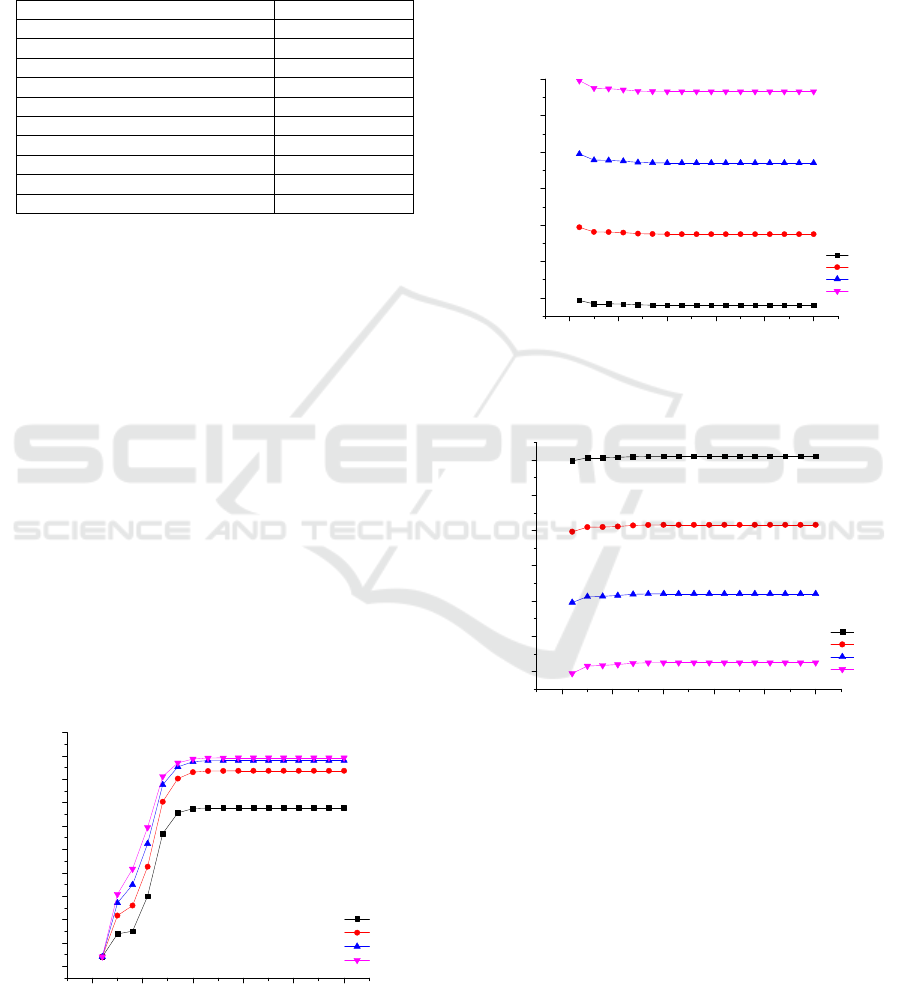
3.1 Sensivity Analysis Results
In this work number of stages, reflux ratio, feed
stage, entrainer feed stage, binary feed stage, and
entrainer mole flow were analyzed. The effect of the
number of stage and reflux ratio to the compositions
of ethanol in the distillate (x
D
) reported in Figure 2.
The higher reflux ratio give the higher purity of the
ethanol in the distillate because more contact
between liquid and vapor occured in the extractive
distillation column. The highest x
D
can be obtained
from the reflux ratio of 2. The ethanol concentration
changed significantly from stage 1 until stage 20
and remains constant at number of stage range from
20 to 50. It show that the extractive distillation
column can be operated in 20 stages and at a reflux
ratio of 2 as an optimal condition.
0 1020304050
0.82
0.84
0.86
0.88
0.90
0.92
0.94
0.96
0.98
1.00
1.02
xD
Number of Stage
RR: 0.5
RR: 1
RR: 1.5
RR: 2
Figure 2: The effect of the number of stages and reflux
ratio to the ethanol composition in the distillate.
The effect of the number of stages and reflux
ratio to the reboiler and condensor duty were
analyzed in Figure 3 and Figure 4, respectively. The
number of stages did not change the duties for both
cases, but the relux ratio give the significant effect. It
means that the extractive distillation column energy
consumption was influenced by the relux ratio. From
these results, it can be concluded that the optimal
reflux ratio is 2 which can save the energy
consumption.
0 1020304050
350000
400000
450000
500000
550000
600000
650000
Reboiler Duty (cal./sec.)
Number of Stage
RR:0.5
RR:1
RR:1.5
RR:2
Figure 3: The effect of the number of stages and reflux
ratio to the reboiler duty.
0 1020304050
-600000
-550000
-500000
-450000
-400000
-350000
-300000
Condensor duty (cal./sec.)
Number of Stage
RR:0.5
RR:1
RR:1.5
RR:2
Figure 4: The effect of the number of stages and reflux
ratio to the condensor duty.
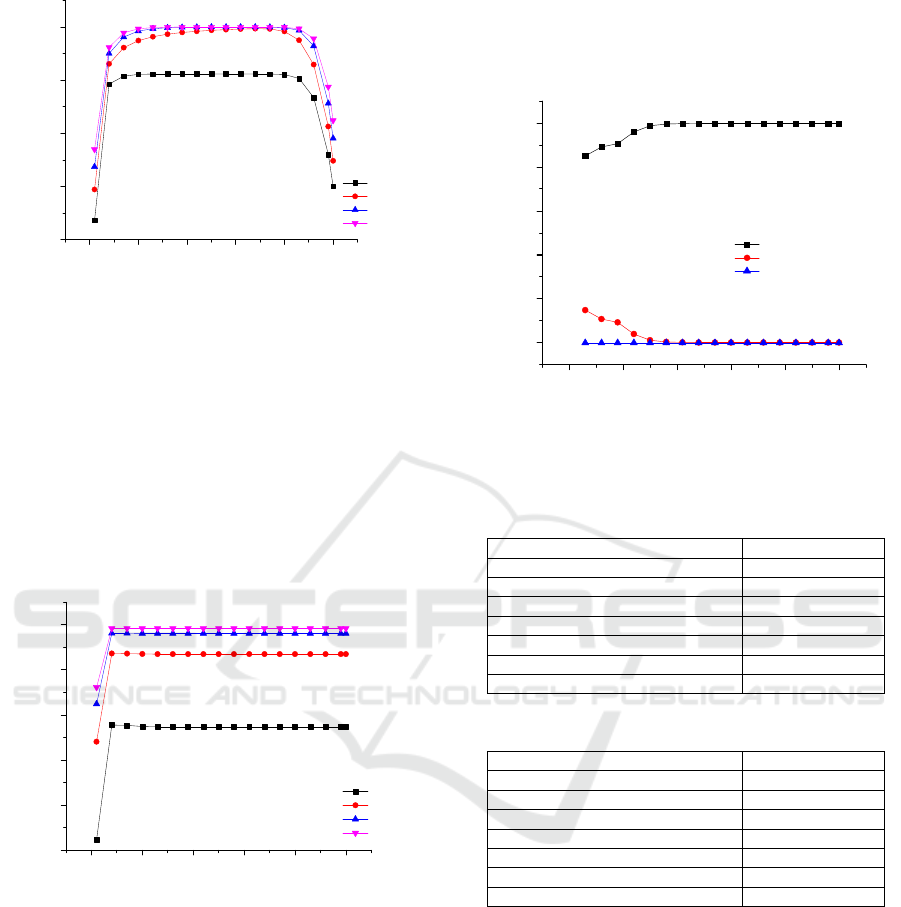
The effect of binary feed stage and reflux ratio to
the ethanol composition in the distillate is shown in
Figure 5. The binary feed stage give the best result in
stages 13 to 40 with the reflux ratio of 2. These
stages give longer and optimal contact between feed
and entrainer. The decreasing of the ethanol
composition occured in stages 40 to 50 because in
this stages, feed position is near the bottom and
reboiler thus it have high possibility become vapor
phase.
EIC 2018 - The 7th Engineering International Conference (EIC), Engineering International Conference on Education, Concept and
Application on Green Technology
452
0 1020304050
0.80
0.85
0.90
0.95
1.00
xD
Binary Feed Stage
RR: 0.5
RR: 1
RR: 1.5
RR: 2
Figure 5: The effect of the binary feed stage and reflux
ratio the ethanol composition in the distillate.
Figure 6 shows the entrainer feed stage reach the
optimal results at stage 3. It is reported that the
highest ethanol composition in the distillate were
obtained at reflux ratio of 2. The ethanol composition
was constant at entrainer feed stages from 3-50
because the best interaction between feed and
entrainer occured in a liquid phase. The entrainer
predominantly in liquid phase when fed in the top
and all stages below the top. Entrainer tend to vapor
phase when it fed in the bottom.
0 1020304050
0.90
0.92
0.94
0.96
0.98
1.00
xD
Entrainer Feed Sta
g
e
RR: 0.5
RR: 1
RR: 1.5
RR: 2
Figure 6: The effect of the entrainer stage and reflux ratio
the ethanol composition in the distillate.
3.2 Simulation Results
Two columns are involved in the extractive
distillation simulation to separate ethanol from water
using ethylene glycol as an entrainer. The first
column was the extractive distillation column which
produced ethanol with purity of 99.8% (mole
fraction). The second column was ethylene glycol
recovery column which the purity of ethylene glycol
can be recovered was 99.6% (mole fraction). The
comparison the effect of the number of stages to the
mole fraction of ethanol, water, and ethylene glycol
was shown in Figure 7. The optimum configuration
and operating conditions obtained in the simulation
for the extractive distillation and recovery column are
shown in Tables 5 and 6.
0 1020304050
0.0
0.2
0.4
0.6
0.8
1.0
Mole Fraction
Number of Stage
xD (ethanol)
xB (water)
xB (ethylene glycol)
Figure 7: The effect of the number of stages to ethanol
composition in the distillate, and water and ethylene
glycol compositions in the bottom.
Table 5: Extractive Distillation Column Design.
Parameters Value
Number of stage 23
Binary feed stage 13
Entrainer feed stage 5
Reflux ratio 2
Entrainer molar ratio 0.059
Entrainer temperature (°C) 25
Distillate mole flow (kmol/h) 78.28
Table 6: Recovery Column Design.
Parameters Value
Number of stage 17
Distillate mole flow (kmol/h) 18.8
Feed stage 12
Reflux ratio 1
Bottom temperature (°C) 469.48
Distillate temperature (°C) 99.25
Distillate mole flow (kmol/h) 18.8
4 CONCLUSIONS
The simulation show the optimal operating condition
to separate the azeotropic mixture of ethanol and
water using ethylene glycol as an entrainer. The
sensivity analysis were conducted to obtain the best
condition and configuration for the extractive
distillation column and recovery column. The
composition of high purity of ethanol and energy
required were consistent. Ethylene glycol is one of
the suitable entrainer which can be used to separate
Simulation of the Extractive Distillation using Ethylene Glycol as an Entrainer in the Bioethanol Dehydration
453

the azeotrope in ethanol-water mixture to obtain
high grade bioethanol.
ACKNOWLEDGEMENTS
This research is fully supported by DIPA Universitas
Negeri Semarang Grant, No. 042.01.2.400899/2018.
REFERENCES
Anisuzzaman, S. M., Krishnaiah, D., Bono, A., Lahin, F.
A., Suali, E. & Zuyyin, I. A. Z, 2018. ‘Simulation and
Optimisation of Bioethanol Purification using
Extractive Distillation with Additive Solvent’,
Pertanika J. Sci. & Technol, Vol. 26, No.2, pp. 707–
718.
Black, C. & Ditsler, D., 1972. ‘Dehydration of aqueous
ethanol mixtures by extractive distillation’, Extractive
and Azeotropic Distillation, pp. 1–15.
Dias, M. O. S., Junqueira, T. L., Maciel Filho, R., Maciel,
M. R. W. & Eduardo Vaz Rossell, C., 2009.
"Anhydrous bioethanol production using bioglycerol -
simulation of extractive distillation processes",
Computer Aided Chemical Engineering. Elsevier B.V.,
Vol. 26, pp. 519-524.
Frolkova, A. K. & Raeva, V. M., 2010. ‘Bioethanol
dehydration: State of the art’, Theoretical Foundations
of Chemical Engineering, Vol.44, No.4, pp. 545–556.
Fu, J., 2004. ‘Simulation of Salt-Containing Extractive
Distillation for the System of
Ethanol/Water/Ethanediol/KAc. 1. Calculation of the
Vapor−Liquid Equilibrium for the Salt-Containing
System’, Industrial & Engineering Chemistry
Research. American Chemical Society, Vol. 43, No. 5,
pp. 1274–1278.
Gil, I. D., García, L. C. & Rodríguez, G., 2014.
‘Simulation of ethanol extractive distillation with
mixed glycols as separating agent’, Brazilian Journal
of Chemical Engineering, Vol. 31, No.1, pp. 259–270.
Gil, I. D., Uyazán, A. M., Aguilar, J. L., Rodríguez, G. &
Caicedo, L. A., 2008. ‘Separation of ethanol and water
by extractive distillation with salt and solvent as
entrainer: Process Simulation’, Brazilian Journal of
Chemical Engineering, Vol. 25, No. 1, pp. 207–215.
Hardjono, Mustain, A., Suharti, P. H., Hartanto, D. &
Khoiroh, I., 2017. ‘Isobaric vapor-liquid equilibrium
of 2-propanone+2-butanol system at 101.325 kPa:
Experimental and molecular dynamics simulation’,
Korean Journal of Chemical Engineering. Vol. 34,
Issue 7, pp 2011–2018.
Hartanto, D., Gupta, B. S., Taha, M. & Lee, M.-J., 2016.
‘Isobaric vapour–liquid equilibrium of (tert-
butanol+water) system with biological buffer TRIS at
101.3kPa’, The Journal of Chemical
Thermodynamics,Vol. 98, pp. 159–164.
Hartanto, D., Mustain, A. & Nugroho, F. D., 2017.
‘Prediction of vapor-liquid equilibria for the alcohol +
glycerol systems using UNIFAC and modified
UNIFAC (Dortmund)’, AIP Conference Proceedings.
Vol. 1818, No. 1, pp. 020017
Kamihama, N., Matsuda, H., Kurihara, K., Tochigi, K. &
Oba, S., 2012. ‘Isobaric Vapor–Liquid Equilibria for
Ethanol + Water + Ethylene Glycol and Its Constituent
Three Binary Systems’, Journal of Chemical &
Engineering Data. American Chemical Society, Vol.
57, No. 2, pp. 339–344.
Kiss, A. A. & Suszwalak, D. J.-. P. C., 2012. ‘Enhanced
bioethanol dehydration by extractive and azeotropic
distillation in dividing-wall columns’, Separation and
Purification Technology, Vol. 86, pp. 70–78.
Seo, D.-J., Takenaka, A., Fujita, H., Mochidzuki, K.&
Sakoda, A., 2018. ‘Practical considerations for a
simple ethanol concentration from a fermentation
broth via a single adsorptive process using molecular-
sieving carbon’, Renewable Energy, Vol. 118, pp.
257–264.
Xu, Y. M., Tang, Y. P., Chung, T.-S., Weber, M. &
Maletzko, C., 2018. ‘Polyarylether membranes for
dehydration of ethanol and methanol via
pervaporation’, Separation and Purification
Technology, Vol. 193, pp. 165–174.
Yeh, A. I. & Berg, L., 1992. ‘The Dehydration of Ethanol
by Extractive Distillation’, Chemical Engineering
Communications, Vol. 113. No.1, pp. 147–153.
Zabed, H., Sahu, J. N., Suely, A., Boyce, A. N. & Faruq,
G., 2017. ‘Bioethanol production from renewable
sources: Current perspectives and technological
progress’, Renewable and Sustainable Energy
Reviews. Pergamon, Vol 71, pp. 475–501.
Zhu, Z., Ri, Y., Li, M., Jia, H., Wang, Y. & Wang, Y.,
2016. ‘Extractive distillation for ethanol dehydration
using imidazolium-based ionic liquids as solvents’,
Chemical Engineering and Processing: Process
Intensification. Elsevier B.V., Vol. 109, pp. 190–198.
EIC 2018 - The 7th Engineering International Conference (EIC), Engineering International Conference on Education, Concept and
Application on Green Technology
454
