
Microstructure and Physical of Al
2
O
3
2SiO
4
2H
2
O Kaolinite Particle
Analysis by Shacking Time and Powder Metallurgy
Agus Nugroho
1
, Basyirun
1
, Rizalman Mamat
2
, Januar Parlaungan Siregar
2
,
Dwi Widjanarko
1
,
Ramelan
1
,
1
Department of Mechanical Engineering, Universitas Negeri Semarang, Semarang, Indonesia
2
Department of Mechanical Engineering, Universiti Malaysia Pahang, Pahang, Malaysia
dwi2_oto@mail.unnes.ac.id, ramelan@mail.unnes.ac.id
Keywords: Kaolinite Material Analysis, Nanoparticle, Nanotechnology, Shacking Time.
Abstract: The shacking time effect on the kaolinite material morphology, physical and grain size have been analyzed.
The aim of this study was to synthesize kaolinite material from Indonesian kaolin to gain nanoscale particle
reduction by shacking time and powder metallurgy cycle. Kaolinite particle powder was successfully
synthesized from thick kaolin solution. The increase in the shacking time resulted in the homogenous
dispersion of kaolinite particles, the reduction of grain size particle clustering, and the reduction of distances
between its particles. The significant grain refining during shacking was revealed which showed reduction
of particle size resulting from longer milling time. SEM analysis of the nanoparticle explained that
designated ball shacking presents to the crystalline refining development. It can be discussed that these
morphological and microstructural variations of Al2O
3
2SiO
4
2H
2
O particle powders developed by
designated ball shacking time were found to present to an improvement in the density, grain refinery, grain
size of kaolinite material particle. The modification of particle grain size is possible into an initial nanoscale
by employing shacking and milling powder metallurgy process. A significant reduction of grain size
reduction was acquired in all cases.
1 INTRODUCTION
Kaolin’s group minerals and its derivative metaform
is characterized by a rather simple chemical
composition of Si, Al, O, and H (Dill, 2016). Kaolin
is relatively pure clay and it has been widely used in
ceramic industries for years (Chen, Lan and Tuan,
2000). The main chemical elements of kaolin, is a
hydrous aluminum silicate of the approximate
composition 2H
2
0-A1
2
O
3
-2SiO
2
. Structurally,
kaolinite material consists of alumina octahedral
sheets and silica tetrahedral sheets stacked
alternately and has the theoretical composition
46.54% Si, 39.5°/0 A12O
3
, 13.96% H2O (Prasad,
Reid and Murray, 1991).
The main mineral component of kaolin is
kaolinite, which consists of layers held together
through hydrogen bonds. Each layer consists of a
two-dimensional arrangement of Al-centred
octahedral (O) and a two-dimensional arrangement
of Si-centred tetrahedral (T) (Zsirka et al., 2015).
The layer is formulated of tetrahedral (Si-O) and
octahedral (Al-O) films bonded through common
oxygen. Moreover, kaolinite occupies a unique
asymmetric interlayer arrangement with two
chemically different surfaces: oxygen of the
tetrahedral film and inner surface hydroxyls of the
octahedral film (Matusik and Matykowska, 2014).
This enables for the synthesis of new nanomaterial
products with precisely defined properties (Matusik
and Bajda, 2013).
Kaolinite is a refractory material since it is a
non-metallic material capable of enduring high
temperatures and suitable as construction materials
for industrial furnaces. Their primary purpose is
derived from their resistance to high temperatures
(Aramide and Seidu, 2013). One of its application is
for refractory metal furnace material. However, to
develop reliable metal furnace it requires optimum
material alloy and optimum grain size.
The particular grain size of the material
generates different atomic bonding (Taylor,
Meyersm and Ashworth, 2007). In the term of
material selection that process would affect micro-
48
Nugroho, A., Basyirun, ., Mamat, R., Siregar, J., Widjanarko, D. and Ramelan, .
Microstructure and Physical of Al2O3 2SiO4 2H2O Kaolinite Particle Analysis by Shacking Time and Powder Metallurgy.
DOI: 10.5220/0009006200480053
In Proceedings of the 7th Engineering International Conference on Education, Concept and Application on Green Technology (EIC 2018), pages 48-53
ISBN: 978-989-758-411-4
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

structure and which determines the properties of the
material (Callister Jr and Rethwisch, 2009). There-
fore the aim of this work is to prepare AL
2
O
3
2SIO
4
2H
2
O particle using mechanical shacking milling
method in order to reduce the existing grain size to
nanoscale particle reduction, dispersion, and mor-
phology of particles. Through milling process, it is
possible to acquire solid materials with wider sur-
face area and different particle sizes (Leonel, 2014).
2 EXPERIMENTAL
2.1 Material
For the preparation of the microstructures, four
samples of Indonesian kaolin were processed. The
untreated kaolin was measure in the ground is
2.24µm. The solvent used: ethanol 70%. The
chemical compositions of the raw kaolin are given in
Table 1, 2, 3 and 4. This chemical composition
acquired by SEM EDX testing from the untreated
sample. The test given in four different spots to get
the average chemical composition of the sample.
2.2 Kaolinite Particle Synthesis
The kaolin powder was purchased from the local
chemical store in Semarang and placed in a plastic
bag prior to the measurement of its weight. 9 grams
of kaolin sample was prepared in a separated
container prior to the treatment. The kaolin powder,
ethanol and ball mill were placed in the stainless
chamber of the shacking mill machine. It was then
shacked and milled in eight axes to optimize its
kaolinite particle fabrication in 30, 60 and 90
minutes individually with ethanol solution
maintaining a liquid ratio of kaolin and ball mill
1:10 by mass. The thick solution was collected for
the synthesis of kaolinite particle. The sample was
handled for drying purpose and moved from the
chamber into a separated container prior
characterization sample preparation. A standardize
coating was done prior to the sample charac-
terization process to investigate its microstructure.
2.3 Structural Characterization
Method
Scanning Electron Microscopy (SEM) - Energy
Dispersive X-Ray (EDX) analyzer from
PhenomProx was employed for structural
characterization of the samples to determine the
chemical elements, microstructure, dispersion,
morphology, and its grain size. Each sample was
placed on a carbon type and coated by Aurum in
18mA for optimal result. Untreated sample as the
first sample was analyzed by Scanning Electron
Microscopy (Choi et al., 2016) and Energy
Dispersive X-Ray (EDX) analyzer (Bhattacharyya
and Behera, 2017).
However, the other three sample which has
been treated were analyzed by Scanning Electron
Microscopy (Takeda et al., 2013) since there is no
additional chemical element process during the
particle synthesis process. The result of each
characterization shown in Figure 2 to 9.
3 RESULT AND DISCUSSION
It is acknowledged that milling processes of
crystalline material enable a significant change in
the morphology of powders as a result of great
plastic deformation of the particles within the
milling process (Hossein-zadeh and Razavi, 2013).
3.1 Particle Chemical Composition
The kaolinite particle chemical composition analysis
was taken from four different spots as shown in
Figure 1.
Figure 1: Scanning Electron Microscopy of untreated
kaolinite material morphology.
Table 1: Kaolin particle chemical composition in spot 1.
Element
Weight Percentage
O
59.3%
Al
16.1%
Si
13.7%
Sr
10.0%
Ba
0.9%
K
0%
Microstructure and Physical of Al2O3 2SiO4 2H2O Kaolinite Particle Analysis by Shacking Time and Powder Metallurgy
49

Table 2: Kaolin particle chemical composition in spot 2.
Element
Weight Percentage
O
61.0%
Al
14.8%
Si
15.9%
Sr
8.3%
K
0%
Table 3: Kaolin particle chemical composition in spot 3.
Element
Weight Percentage
O
57.4%
Al
16.0%
Si
13.9%
Sr
9.6%
K
3.1%
Table 4: Kaolin particle chemical composition in spot 4.
Element
Weight Percentage
O
58.4%
Al
16.3%
Si
14.0%
Sr
10.7%
Ba
0.6%
Figure 1 shows Scanning Electron Microscopy
of untreated kaolinite material morphology, the
chemical composition testing was done in four
different spots. It is possible to examine the detail
chemical composition as shown in table 1, 2, 3 and
4. Major chemical compositions of the sample are
Oxygen, Aluminium and Silica are visible in a
significant amount. However, there is a phenomenon
that the sample has Strontium, Barium, and Kalium
at spot number 3. It can be discussed as the
uniqueness of the Indonesian kaolin chemical
composition influenced by the natural development
in the soil.
3.2 Particle Microstructure
The micro kaolinite particles were synthesized and
its morphology and dispersion are shown in Figures
below. Figure 2 describes the microstructure,
morphology and kaolinite particle dispersion of the
untreated sample in 5,000x magnitude and 10µm
length. While Figure 3 describes the microstructure,
morphology and kaolinite particle dispersion of the
untreated sample in 20,000x magnitude and 5µm
length. The grain size was investigated that it is still
possible to perceive the presence of crystalline
materials with a relatively big size throughout the
surface of the sample.
Figure 2: Scanning Electron Microscopy of untreated
kaolinite material.
Figure 3: microstructure, morphology and kaolinite
particle .
The grain size investigation was done in five
different spots as shown in Figure 2 above. The
initial diameter of grain size was investigated, it is
2.24µm. The results examined in Figure 4 and 5
describe the existence of different morphological
transformation phenomena which occur
simultaneously along with the shacking process
(Leonel et al., 2014). It can be explained that the
particular particles initiated to crack due to the
shacking mechanism. As a matter of fact that
kaolinite material is a brittle material, the breakage
of it is particle occurs in a wide dispersion
throughout the particle surface as shown in Figure 4
and 5.
EIC 2018 - The 7th Engineering International Conference (EIC), Engineering International Conference on Education, Concept and
Application on Green Technology
50
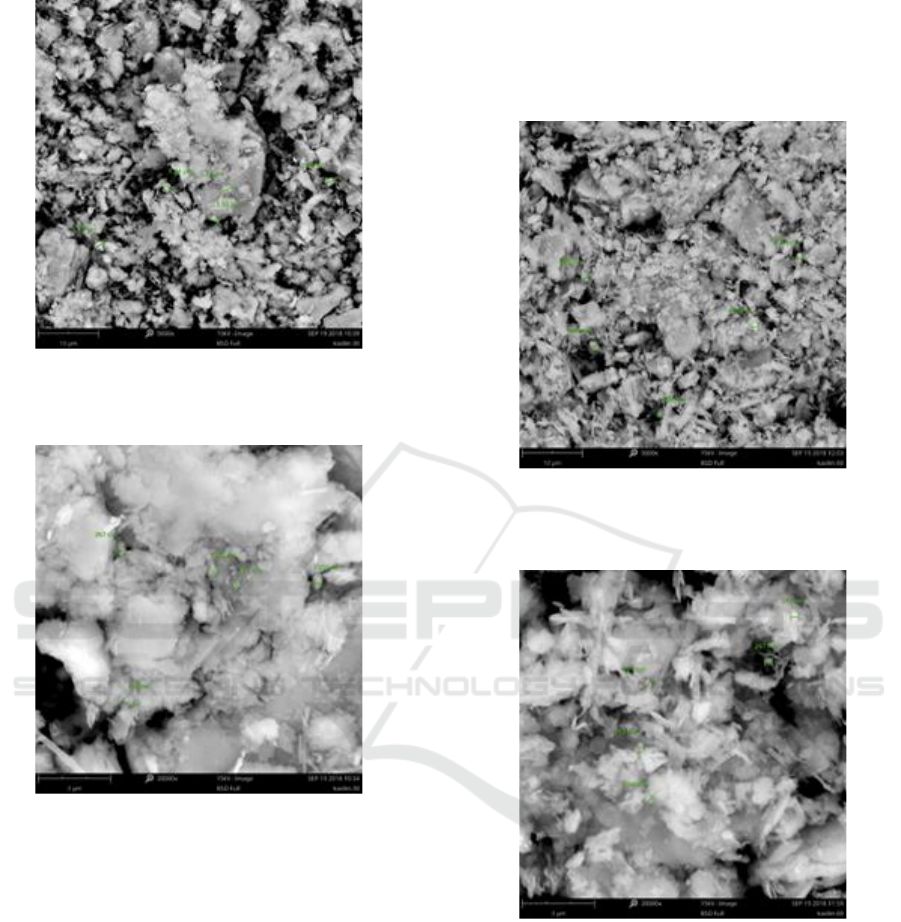
Figure 4: Scanning Electron Microscopy of kaolinite
material morphology after 30 minutes of treatment.
Figure 5: Scanning Electron Microscopy of kaolinite
material grain size after 30 minutes of treatment.
It is explained that the dispersion of its
morphology of the particle is wider, the pores
visibility of the particles are relatively reduced. This
means there is a reduction of the particles into a
smaller grain size. As the result, there is an
escalation of the surface contact area of each
particle. It shows on Figure 6 that the grain size
particles range is between 990nm - 1.07µm. More
detail result can be obtained from Figure 7 which
explains that the grain size range is between 312 –
297nm. Through both Figure 8 and 9 it can be
examined that there is a shrinkage in the width of the
peak demonstrating the formation of a slightly more
homogeneous particle in comparison with the
material before the treatment process (Hubadillah et
al., 2016). There is a significant grain size
decrement subject to each particle of the kaolinite
material. It shows that the morphology of the
material is more homogenous and the pores number
are decreased tremendously. The diameter of grain
size obtained in the range of 211 – 190nm.
Figure 6: Scanning Electron Microscopy of kaolinite
material morphology after 60 minutes of of treatment.
Figure 7: Scanning Electron Microscopy of kaolinite
material grain size after 60 minutes of of treatment.
Furthermore, it can be investigated further from
Figure 9 that the surface area of contact is larger
than the previous ones. Besides, AL2O
3
2SiO
4
2H
2
O
nanoparticles materialize in large arrays initiate to
disperse within kaolinite particles with a better
homogeneity and smaller area by escalating the
shacking life (Toozandehjani et al., 2017). The
significant disparity in the crystalline size and in the
lattice strain of kaolinite particle is associated to
great plastic deformation and grain size refinement
Microstructure and Physical of Al2O3 2SiO4 2H2O Kaolinite Particle Analysis by Shacking Time and Powder Metallurgy
51
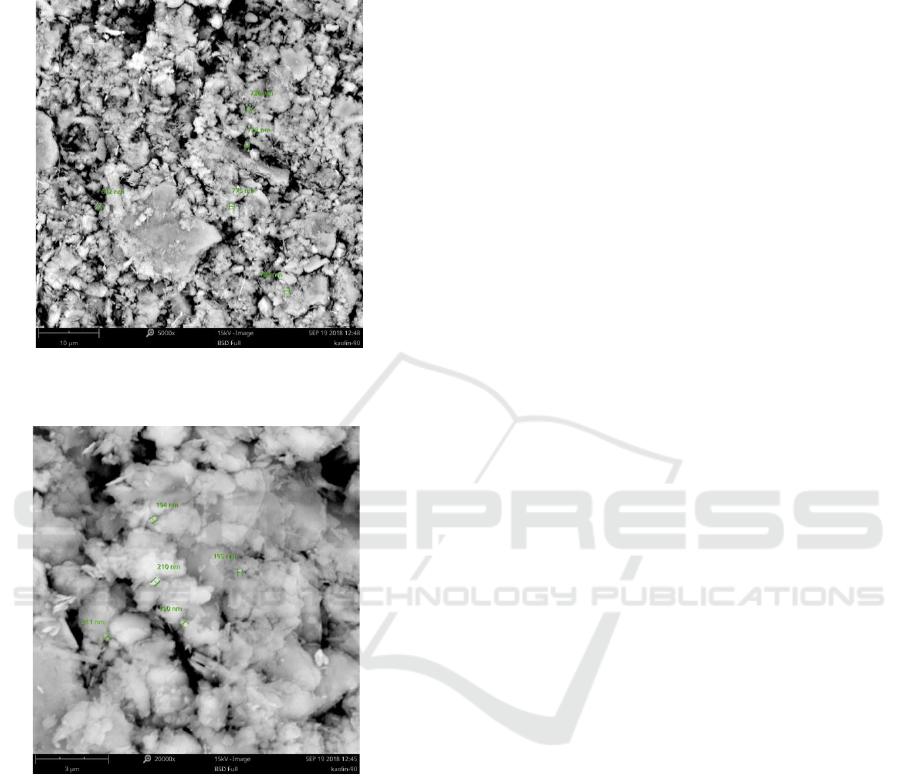
occurring in particles in the presence of AL2O
3
2SiO
4
2H
2
O initial nanoparticles as the main
subscription to the shacking cycle (Mobasherpour,
To and Ebrahimi, 2013).
Figure 8: Scanning Electron Microscopy of kaolinite
material morphology after 90 minutes of treatmen.
Figure 9: Scanning Electron Microscopy of kaolinite
material grain size after 60 minutes of treatment.
4 CONCLUSIONS
Kaolinite particle powder was successfully
synthesized from thick kaolin solution by shacking
and milling of power metallurgy process. The
shacking-milling mechanism was a crucial step for
generating the quality product of particle. The initial
grain size of the untreated sample range is 2.24µm to
660nm. Furthermore, nanoparticles appear in large
clusters start to disperse within kaolinite particles
with a better homogeneity after 90 minutes of
treatment. As the result, the grain size diameter has
been reduced to 190nm. The by-product initial
nanoparticle hydrous alumina-silica of material
formed in this process is a non-hazardous material.
Thus, we can convey the process followed in this
paper is an accessible, affordable and environment-
friendly method for kaolinite particle synthesis from
Indonesia kaolin. The modification of particle grain
size is possible into a nanoscale by employing
shacking and milling powder metallurgy process. A
significant of grain size reduction was acquired in all
cases.
ACKNOWLEDGMENTS
The researchers would like to thank the Faculty of
Engineering, Semarang State University, Indonesia
for the funding research, to a Physic Laboratory for
SEM characterization and Faculty of Mechanical
Engineering, Universiti Malaysia Pahang, Malaysia
for the great support during the research process.
REFERENCES
Aramide, F. O. & Seidu, S. O. 2013. ‘Production of
Refractory Lining for Diesel Fired Rotary Fur-
nace, from Locally Sourced Kaolin and Potter’s
Clay’, Journal of Minerals and Materials
Characterization and Engineering, 1(3), pp. 75–
79. doi: 10.4236/jmmce.2013.13014.
Bhattacharyya, S. & Behera, P. S., 2017.‘Applied
Clay Science Synthesis and characterization of
nano-sized α -alumina powder from kaolin by
acid leaching process’, Applied Clay Science.
Elsevier, 146(June), pp. 286–290. doi:
10.1016/j.clay.2017.06.017.
Callister Jr, W. D. & Rethwisch, D. G., 2009.
Material Science and Engineering : An
Introduction. Eight. John Wiley & Sons, Inc.
Chen, C. Y., Lan, G. S. & Tuan, W. H., 2000.
‘Microstructural evolution of mullite during the
sintering of kaolin powder compacts’, Ceramics
International, 26(7), pp. 715–720. doi:
10.1016/S0272-8842(00)00009-2.
Choi, Y., Choo, H., Yun, T. S., Lee, C. & Lee, W.
(2016) ‘Engineering characteristics of chemically
treated water-repellent Kaolin’, Materials, 9(12).
doi: 10.3390/ma9120978.
Dill, H. G., 2016 ‘Kaolin: Soil, rock and ore: From
the mineral to the magmatic, sedimentary and
metamorphic environments’, Earth-Science
EIC 2018 - The 7th Engineering International Conference (EIC), Engineering International Conference on Education, Concept and
Application on Green Technology
52

Reviews. Elsevier B.V., 161, pp. 16–129. doi:
10.1016/j.earscirev.2016.07.003.
Hossein-zadeh, M. & Razavi, M., 2013. ‘Charac-
terization of properties of Al – Al 2 O 3 nano-
composite synthesized via milling and
subsequent casting’, Journal of King Saud
University - Engineering Sciences. King Saud
University, 25(1), pp. 75–80. doi:
10.1016/j.jksues.2012.03.001.
Hubadillah, S. K., Harun, Z., Othman, M. H. D.,
Ismail, A. F. & Gani, P., 2016. ‘Effect of kaolin
particle size and loading on the characteristics of
kaolin ceramic support prepared via phase
inversion technique’, Journal of Asian Ceramic
Societies. Taibah University, 4(2), pp. 164–177.
doi: 10.1016/j.jascer.2016.02.002.
Leonel, E. C., Nassar, E. J., Ciuffi, K. J., Reis, M. J.
dos & Calefi, P. S., 2014. ‘Effect of high-energy
ball milling in the structural and textural
properties of kaolinite’, Cerâmica, 60(354), pp.
267–272. doi: 10.1590/S0366-691320140002000
16.
Matusik, J. & Bajda, T., 2013. ‘Journal of Colloid
and Interface Science Immobilization and
reduction of hexavalent chromium in the
interlayer space of positively charged kaolinites’,
Journal of Colloid And Interface Science.
Elsevier Inc., 398, pp. 74–81. doi:
10.1016/j.jcis.2013.02.015.
Matusik, J. & Matykowska, L., 2014. ‘Behaviour of
kaolinite intercalation compounds with selected
ammonium salts in aqueous chromate and
arsenate solutions’, JOURNAL OF
MOLECULAR STRUCTURE. Elsevier B.V. doi:
10.1016/j.molstruc.2014.04.063.
Mobasherpour, I., To, A. A. & Ebrahimi, M., 2013.
‘Effect of nano-size Al 2 O 3 reinforcement on
the mechanical behavior of synthesis 7075
aluminum alloy composites by mechanical
alloying’, 138. doi: 10.1016/j.matc
hemphys.2012.12.015.
Prasad, M. S., Reid, K. J. & Murray, H. H., 1991.
‘Kaolin : processing , properties and appli
cations’, 6, pp. 87–119.
Takeda, H., Hashimoto, S., Yokoyama, H., Honda,
S. & Iwamoto, Y., 2013. ‘Characterization of
zeolite in zeolite-geopolymer hybrid bulk
materials derived from kaolinitic clays’,
Materials, 6(5), pp. 1767–1778. doi:
10.3390/ma6051767.
Taylor, P., Meyersm, M. A. & Ashworth, E., 2007.
‘A model for the effect of grain size on the yield
stress of metals’, (March 2015), pp. 37–41. doi:
10.1080/01418618208236928.
Toozandehjani, M., Matori, K., Ostovan, F., Abdul
Aziz, S. & Mamat, M., 2017. ‘Effect of Milling
Time on the Microstructure, Physical and
Mechanical Properties of Al-Al2O3 Nano-
composite Synthesized by Ball Milling and
Powder Metallurgy’, Materials, 10(11), p. 1232.
doi: 10.3390/ma10111232.
Zsirka, B., Horváth, E., Makó, É., Kurdi, R. &
Kristóf, J. , 2015. ‘Preparation and charac-
terization of kaolinite nanostructures: reaction
pathways, morphology and structural order’,
Clay Minerals, 50(3), pp. 329–340. doi:
10.1180/claymin.2015.050.3.06.
Microstructure and Physical of Al2O3 2SiO4 2H2O Kaolinite Particle Analysis by Shacking Time and Powder Metallurgy
53
