
Catalytic Converter based on Titanium Oxide (TiO
2
) to Reduce the
Emission of Carbon Monoxide and Hydrocarbon in Exhaust Gas of
Motor Vehicles
Ahmad Mustamil Khoiron
1
, Samsudin Anis
1
, Masugino
1
, Syahdan Sigit Maulana
1
, Saian Nur Fajri
2
1
Department of Mechanical Engineering, Universitas Negeri Semarang, Semarang, Indonesia
2
National Yunlin University of Science and Tecnology, Yunlin, Taiwan
msyahdan19@yahoo.com, saiannurfajri@gmail.com
Keywords: Catalytic Converter, Titanium Oxide, Emission, Carbon Monoxide, Hydro Carbon
Abstract: Motor vehicle is a transportation which uses petroleum fuels as its energy resource. Crude oil as the raw
materials processed into any kinds of petroleum fuels then used for the motor vehicle. Afterward, fuel is
changed into mechanical energy through the combustion process which also produces the dangerous
pollutant. Aimed to reduce the air pollution which has toxicity from internal combustion engine then used
Catalytic Converter. However, Catalytic Converter has a high price and causing most gasoline vehicles did
not fully use this technology. The case happened because catalytic made from Palladium, Platinum, and
Rhodium. The breakthrough is made by making a ceramic-based catalyst with Titanium Dioxide additive.
The purposes of the research are; (a) to know the reduction of carbon monoxide in the exhaust gas emission
using Titanium Oxide (TiO
2
), (b) to know the reduction of HC in the exhaust gas emission using Titanium
Oxide (TiO
2
), (c) to know the effect of the catalytic converter uses Titanium Oxide (TiO
2
) on engine
performance at 1,000; 1,500; and 2,000. The method used was a quasi-experimental design, which
compared experimentally before and after the exhaust gas through a catalytic converter based on TiO
2
.
1 INTRODUCTION
Motor vehicles are the means of transportation used
to assist human mobility in daily activities. The
number of motor vehicles is increasing every year.
The Statistic Indonesia (BPS) data shows that the
number of motor vehicles in 2013 has reached 104
million units, then in 2014, it has increased to 114
million units, by 2015 the number of motor vehicles
reaches more than 121 million units.
Motor vehicles use petroleum fuels as its
energy resource. Then the fuel is converted into
mechanical energy through the combustion process.
From this combustion process, motor vehicles
produce harmful pollutants. According to Sugiharto
et al. (2016), the total estimated CO pollutant of all
activities is about 686,864 tons per year or 48.6
percent of the total emissions of five pollutants. The
cause of air pollution is about 80 percent comes
from the transport sector, and 20 percent from
industry and domestic waste. Whereas, carbon
emissions from deforestation and forest degradation
are 20 percent.
Pollutant gases that generated by motor
vehicles have a harmful impact on human health and
the environment. According to research conducted
by Hajderi and Bozo (2014) conducted in Albania,
motor vehicle exhaust emissions Increase the risk of
chronic bronchitis, hormonal hyperactivity,
dermatitis, anemia, allergies and tumors in humans
and provide an increasing number of cancer patients
and liver disease that result in death to 400
casualties. Furthermore, vehicle exhaust gas can also
cause acid rain, greenhouse effect, and damage the
ozone layer. So the problem of gas dispose of this
motor vehicle requires severe handling.
Various studies have been undertaken to reduce
the impact and content of emissions, such as with
the use of filter material to reduce the pollutant from
the exhaust gas emissions of these vehicles. Ceramic
materials can be used to absorb HC and CO exhaust
emissions. The results of Sinuhaji (2017), which
uses ceramics as an absorbent material of CO up to
84.62%, and HC up to 71.64%. So, this acceptable
Khoiron, A., Anis, S., Masugino, ., Maulana, S. and Fajri, S.
Catalytic Converter based on Titanium Oxide (TiO2) to Reduce the Emission of Carbon Monoxide and Hydrocarbon in Exhaust Gas of Motor Vehicles.
DOI: 10.5220/0009005700150020
In Proceedings of the 7th Engineering International Conference on Education, Concept and Application on Green Technology (EIC 2018), pages 15-20
ISBN: 978-989-758-411-4
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
15

ceramic form can be used as a reference in reducing
vehicle exhaust pollutants.
Based on the research of Amin and Subri
(2016), the manufacture of emission filter material
from Ceramic Matrix Composite (CMC) material
has been successfully manufactured. Using Titanium
Oxide substances as additives are mixed with some
other additives, mixing with a rotational speed of 64
rpm for 30 minutes and molded with 25 MPa
pressure and 9,500C sintering temperature. The
decreasing performance of CO level, up to 99.67%
volume at 2,000 rpm engine speed. Thus, based on
the description, porous ceramics with additional
TiO2 additives may be used to reduce exhaust
emissions of motor vehicles.
Based on the description of the problem and the
results of previous research, it is necessary to
develop the design and use of materials on the
catalytic con-verter which is useful, efficient and
economical, so that all types of motor vehicles can
use catalytic con-verter technology to overcome the
problems of envi-ronmental pollution that will be
bad for human life.
2 METHOD
This study uses a mixed method, a combination of
research and development with experimental
research. Research produces to produce the right
products in the relevant laboratory and
environmental environments. Four D models are
used as a model of research and development which
consists of 4 stages of development. For valid
results, an experimental time series quasi-
experimental model was used to determine the use
of the designed catalytic converter.
In product development, the catalytic converter
design was designed using Autodesk inventor to
then be simulated using ANSYS Fluent. The
experimental research used: 1) independent variable
was a standard exhaust for a light vehicle without
catalytic converter and catalytic converter designed
with ceramic material with additive Titanium
Dioxide (TiO
2
), 2) control variables were rotation
variation for testing engine performance from 1,000
rpm, 1,500 rpm, and 2,000 rpm, and 3) dependent
variables were engine performance (torque and
power), exhaust emissions (carbon monoxide,
carbon dioxide, and hydrocarbons).
The research design can be described as in the
following figure:
Figure 1: Research design.
Note:
O
1
, O
2
, O
3
, O
4
= testing and measuring exhaust
emission before treatment
O
5
, O
6
, O
7
, O
8
= testing and measuring exhaust
emission after treatment
X = treatment using a designed catalytic converter
The result of carbon monoxide from the
exhaust gas measured using a gas analyzer then
incorporated into the test result table as follows
3. DATA ANALYSIS
Data analysis techniques are used to analyze data
that has been collected during the experimental
process. The data analysis technique used in this
research is the comparison of software with the
product. The design was observed by simulation
Table 1: The measurement instrument of HC and CO.
Revolution (rpm)
Without Catalytic Converter (%) With Catalytic Converter (%)
O
1
O
2
O
3
O
4
O
5
O
6
O
7
O
8
1000 (X
1
)
O
1
. X
1
O
2
. X
1
O
3
. X
1
O
4
. X
1
O
5
. X
1
O
6
. X
1
O
7
. X
1
O
8
. X
1
1500 (X
2
)
O
1
. X
2
O
2
. X
2
O
3
. X
2
O
4
. X
2
O
5
. X
2
O
6
. X
2
O
7
. X
2
O
8
. X
2
2000 (X
3
)
O
1
. X
3
O
2
. X
3
O
3
. X
3
O
4
. X
3
O
5
. X
3
O
6
. X
3
O
7
. X
3
O
8
. X
3
EIC 2018 - The 7th Engineering International Conference (EIC), Engineering International Conference on Education, Concept and
Application on Green Technology
16
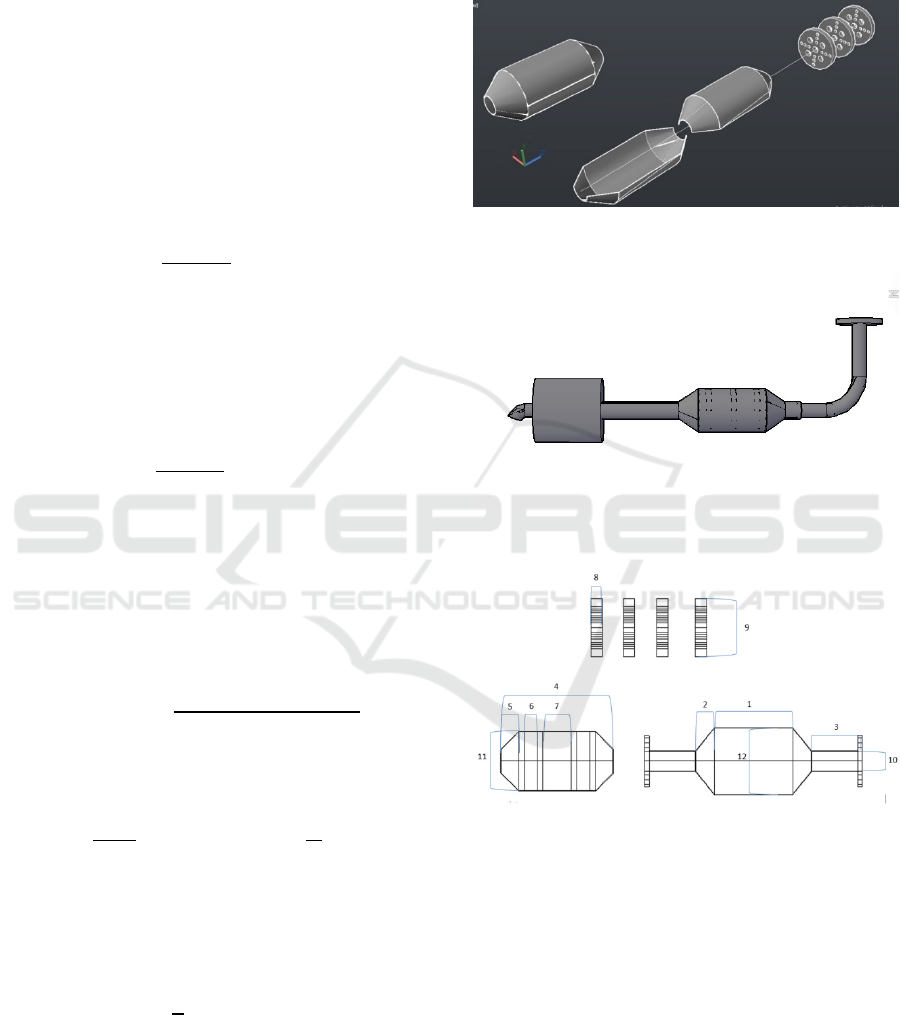
with ANSYS Fluent software, the analysis used was
pressure, temperature, and gas flow rate to be tested
on the catalytic converter made of ceramic with
Titanium Dioxide additive (TiO
2
). Ansys software
will show the pressure, temperature and flow rate on
the inside of the catalytic-based exhaust converter
made from ceramic with Titanium Dioxide additive
(TiO
2
).
The determination of adsorption power of carbon
dioxide (CO) through activated carbon adsorbent,
the effectiveness of adsorbent is measured by
percentage level of adsorbs to exhaust emission and
particulate lead. The formula for measuring the
effectiveness of adsorbents is as follows:
%CO
C1C2
C1
x100%
(1)
The determination of HC reduction power by
reducing ceramic materials, reducing effectiveness is
measured based on percentage reduction rate on HC
exhaust emissions. The formula for measuring the
effectiveness of adsorbents is as follows:
%HC
C1C2
C1
x100%
(2)
Note:
C1 is initial emission levels (without treatment)
C2 is gas emission level after treatment with the
reduction
The determination of exhaust volume through
catalytic converter:
SpaceVelocit
y
Volumeflowrate
Rectorvolume
(3)
where volume flow rate is
.
2
..
2
.60
(4)
and reactor volume, in this case as same as the
volume of the catalytic converter through the
following equation
4
.
.
(5)
4 RESULTS
4.1 Catalytic Converter Design
Figure 2: Catalytic converter in assembly.
Figure 3: Catalytic converter.
Figure 4: Technical part of the catalytic converter.
4.2 Simulation Result
Figure 5 shows the result of the pressure distribution
occurring on the product. The highest value is shown
in red which means that it is 5.761x10
2
, while the
lowest is -6.516x10
1
with blue. As the result of
pressure analysis, the unit used is Pascal (Pa). Figure
9 shows the drop in pressure from the pipe header to
the muffler tip.
Catalytic Converter based on Titanium Oxide (TiO2) to Reduce the Emission of Carbon Monoxide and Hydrocarbon in Exhaust Gas of
Motor Vehicles
17
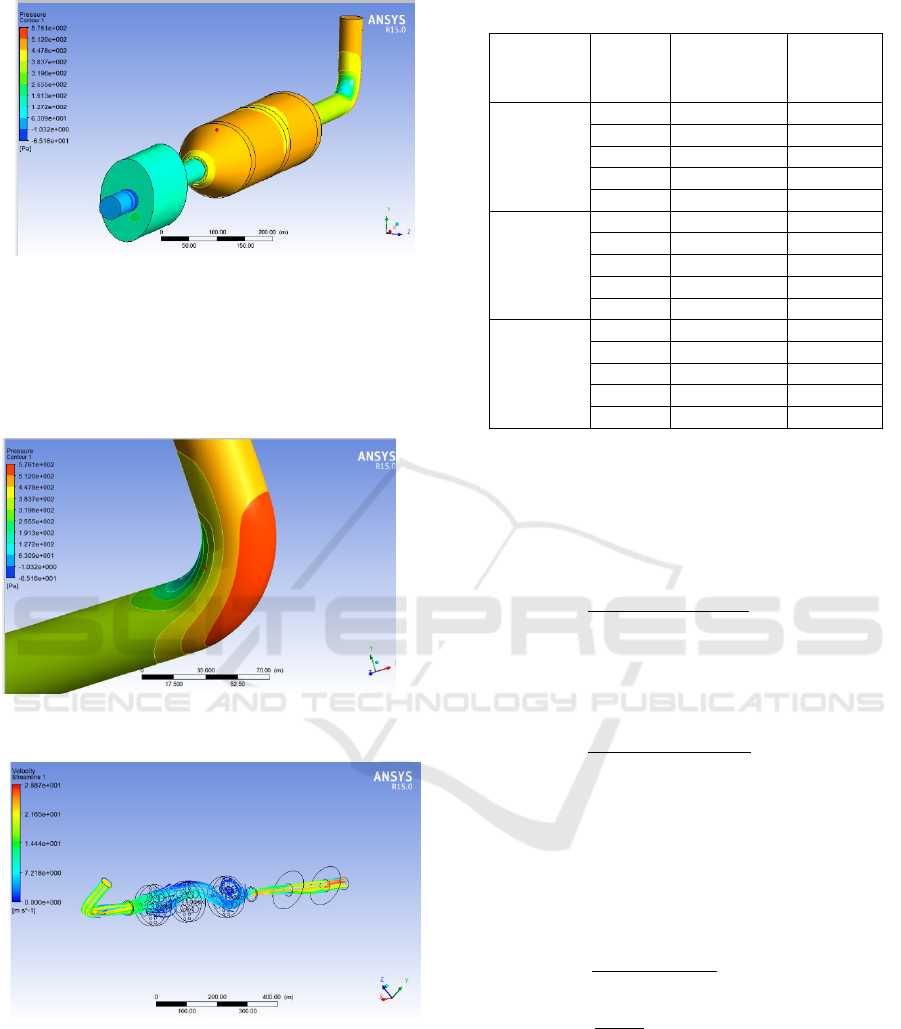
Figure 5: Pressure distribution.
The highest pressure occurs at the bottom
radius of the pipe showing a value of 5.761x10
2
; this
indicates that the part is a critical part because it can
cause material damage due to the pressure that
occurs during the process of working
Figure 6: Pressure Distribution on header pipe.
Figure 7: Fluid flow through the catalytic converter.
4.3 Catalytic Converter Design
Based on the results of catalytic converter testing
using a ceramic catalyst with titanium dioxide
additives to reduce the emission levels of
hydrocarbon carbon monoxide gas shown in the
following table:
Table 2. The Measurement result of HC and CO
Revolution
(rpm)
Exhaust
Gases
Without
Catalytic
Converter
Using
Catalytic
Converter
1,000
CO 3.768% 3.101%
CO2 3.28% 4.03%
HC 111 ppm 140 ppm
O2 13.81% 13.30%
Λ 2.052 2.042
1,500
CO 0.586% 0.162%
CO2 5.40% 5.93%
HC 41 ppm 29 ppm
O2 13.25% 12.97%
Λ 2.467 2.44
2,000
CO 1.193% 1.615%
CO2 5.21% 5.55%
HC 89 ppm 46 ppm
O2 12.87% 12.33%
Λ 2.276 2.069
To calculate the adsorption power or the
decrease of the carbon monoxide and hydrocarbon
content of the catalytic converter design can be used
the following equation, for CO adsorption power
(AB) using:
x100%
(6)
for HC adsorption power formula using:
x100%
(7)
The results of catalytic converter testing using a
ceramic catalyst with titanium dioxide additive at
1000 rpm:
CO before treatment = 3.768 %
CO after treatment = 3.101 %
3.768 3.101
3.768
x100%
0.667
3.768
x100% 17.70%
(8)
The use of the ceramic material as a catalyst
material with the addition of titanium dioxide
additive at 1,000 rpm can reduce carbon monoxide
emission by 17.70% decrease from 3.768%
concentration without catalytic converter to 3.101%.
HC before treatment = 111
HC after treatment = 140
EIC 2018 - The 7th Engineering International Conference (EIC), Engineering International Conference on Education, Concept and
Application on Green Technology
18
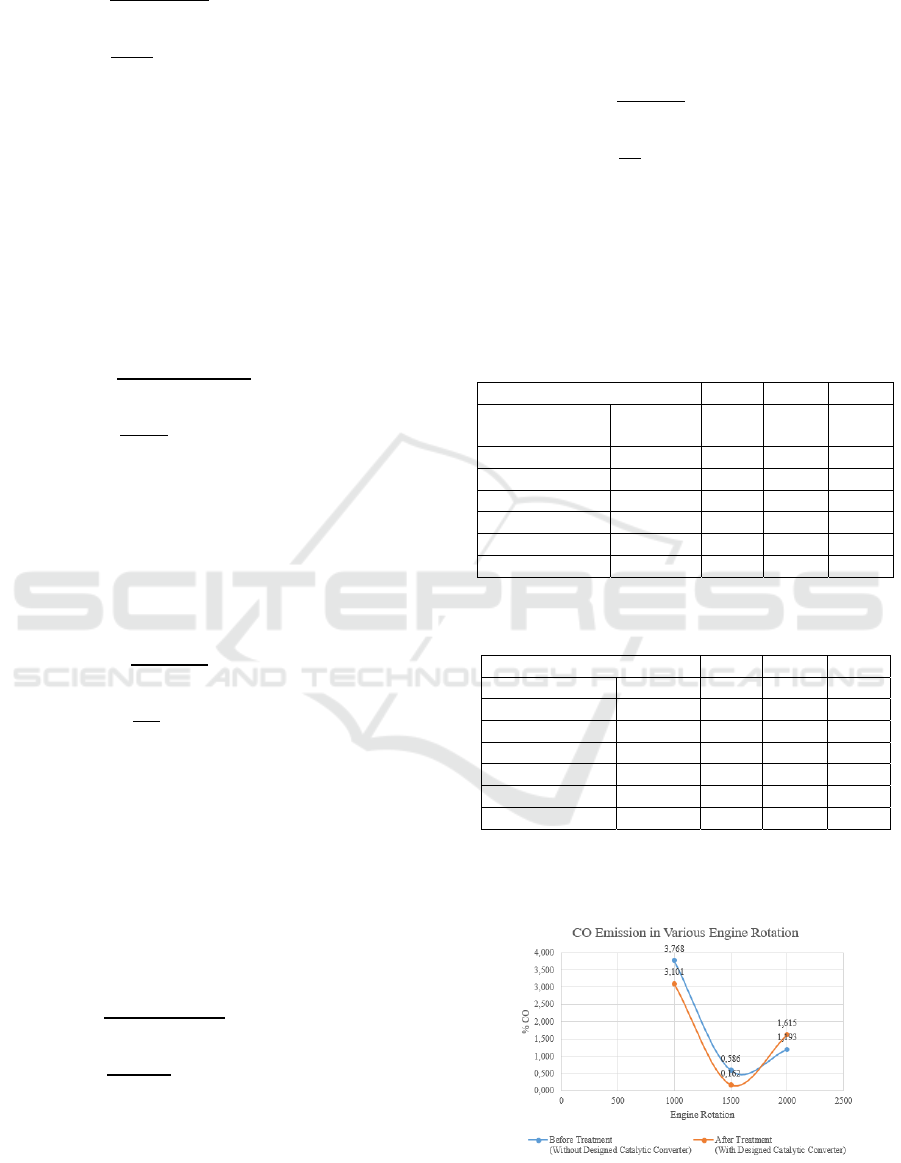
111140
111
x100%
29
111
x100% 26.12%
(9)
The use of the ceramic material as a catalyst
material with the addition of titanium dioxide
additive at 1,000 rpm cannot reduce the emission of
hydrocarbon gas, but increasing 26.12% of the
concentration of 111 ppm without catalytic
converter to 140 ppm.
The results of catalytic converter testing using
ceramic catalyst with titanium dioxide additive at
1,500 rpm:
CO before treatment = 0.586%
CO after treatment = 0.162%
0.5860.162
0.586
x100%
0.424
0.586
x100% 72.35%
(10)
The use of ceramic material as catalyst material
with the addition of additive at 1,500 rpm can reduce
carbon monoxide emission by 72.35% decrease from
0.586% concentration without catalytic converter to
0.162%.
The initial HC content = 41
The final HC content = 29
4129
41
x100%
12
41
x100% 29.26%
(11)
The use of the ceramic material as catalyst
material with the addition of titanium dioxide
additive at 1,500 rpm can reduce the hydrocarbon
emission by 29.26% from 41 ppm concentration
without catalytic converter down to 29 ppm.
The results of catalytic converter testing using a
ceramic catalyst with titanium dioxide additive at
2,000 rpm:
The initial CO content = 1.193 %
The final CO content = 1.615%
1.1931.615
1.193
x100%
0.422
1.193
x100% 35.37%
(12)
The use of the ceramic material as a catalyst
material with the addition of titanium dioxide
additive at 2,000 rpm cannot reduce carbon
monoxide emissions, but increasing 35.37% of the
1.193% concentration without catalytic converter to
1.615%.
The initial HC content = 89
The final HC content = 46
8946
89
x100%
43
89
x100% 50%
(11)
The use of the ceramic material as a catalyst
material with the addition of titanium dioxide
additive at 2,000 rpm can reduce hydrocarbon
emissions by 50% from 89 ppm concentration
without catalytic converter down to 46 ppm.
Table 3: The result of the exhaust emission test without
using a catalytic converter.
Test sequence 1 2 3
Oil
temperature
o
C 122 122 122
Revolution rpm 1,000 1,500 2,000
CO % vol 3.768 0.586 1.193
CO2 % vol 3.28 5.40 5.21
HC ppm vol 111 41 89
O2 % vol 13.81 2.467 12.87
Λ - 2.052 2.467 2.276
Table 4: The result of the exhaust emission test using a
catalytic converter.
Test sequence 1 2 3
Oil temperature
o
C 121 122 122
Revolution rpm 1,000 1,500 2,000
CO % vol 3.101 0.162 1.615
CO2 % vol 4.03 5.93 5.55
HC Ppm vol 140 29 46
O2 % vol 13.30 12.97 12.33
Λ - 2.042 2.445 2.069
5 DISCUSSION
Figure 7: The graph of CO content in emission test with
and without a catalytic converter.
Catalytic Converter based on Titanium Oxide (TiO2) to Reduce the Emission of Carbon Monoxide and Hydrocarbon in Exhaust Gas of
Motor Vehicles
19
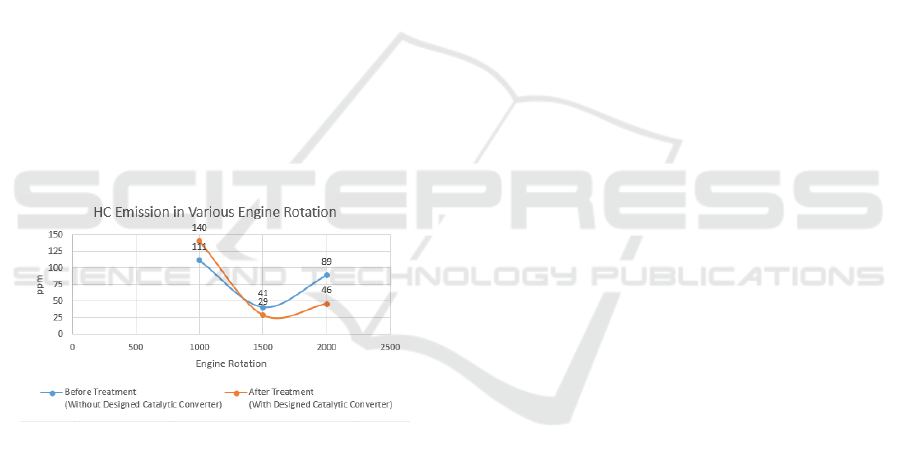
The result of gas emission test of a gasoline engine
in standard condition without using catalytic
converter showed the result of carbon monoxide
concentration 3.768% at 1,000 rpm, 0.586% at 1,500
rpm and 1.193% at 2,000 rpm. While, the resulting
hydrocarbon is 111 ppm at 1,000 rpm, 41 rpm at
1,500 rpm, and 89 ppm at 2,000 rpm. Then on the
exhaust gas emission test using catalytic converter
using porous ceramics with titanium dioxide
additives showed the results of carbon monoxide
concentration of 3.101% at 1,000 rpm, 0.162% at
1,500 rpm, and 1.615% at 2,000 rpm.
The use of a catalytic converter can reduce
carbon monoxide as a whole with an average of
18.22%. Test results look very significant at 1,500
rpm engine speed with a decrease of 72.35%. The
result is due to the effects of the reaction between
titanium dioxide and gasoline exhaust that has a
temperature of 700
o
-1,000
o
C so that it will heat
titanium dioxide up to speed up the reaction. The
porous ceramics present in the catalytic converter
system are capable of capturing and disentangling
the exhaust pollutant of a gasoline engine. The most
effective exhaust gas emission reduction is at 1,500
rpm engine speed, this is due to the engine that uses
the ideal mixed carbu-rettor with stoichiometry is at
medium engine speed.
Figure 8: The graph of HC content in emission test with
and without a catalytic converter.
The use of a catalytic converter can reduce the
overall hydrocarbon level with an average of
17.71%. The most effective reductions occur at
2,000 rpm rotation that is able to reduce the
hydrocarbon level by 50%. The result is due to the
most optimal burning occurs at a speed of 2,000
rpm. On a gasoline engine using a carburetor, 2,000
rpm is classified within the engine speed with the
most optimal fuel consumption.
6 CONCLUSION
The results show that the ability of ceramic
catalytic converter with titanium dioxide additive in
gasoline engine has an average yield of CO emission
reduction with additive titanium dioxide additives
18.22% of a ceramic catalyst. The highest CO
emission reduction is in experimental group 2 that is
72, 35% with 1,500 rpm (medium engine speed).
The average HC emission reduction with a ceramic
catalyst with titanium dioxide additives is 17.71%,
and the highest reduction of HC emission is in
experimental group 3, i.e., 50% with 2,000 rpm.
REFERENCES
Amin, M., & Subri, M. 2016. Karakterisasi Penggunaan
Bahan Absorben dan Katalis Dalam Pembuatan
Material Cmc Untuk Filter Gas Buang Kendaraan
Bermotor. Jurnal Mekanika, 15.
Amin, M., Subri M.. 2016. Uji Performa Filter Gas Emisi
Kendaraan Bermotor Berbasis Keramik Porous
Dengan Aditif Tembaga, TiO
2
Dan Karbon Aktif
Dalam Penurunan Kadar Gas Carbon Monoksida.
Semarang: Universitas Muhammadiyah Semarang
Badan Pusat Statistik. 2015. Perkembangan Jumlah
Kendaraan Bermotor Menurut Jenis, 1949-2015.
Jakarta: Badan Pusat Statistik (https://www.bps.go.id
/linkTableDinamis/view/id/1133: accessed on 3 Mei
2018)
Hajderi, A., Bozo L. 2014. Air Pollution from Vehicles
and Their Effect on Human Health in Urban Areas.
Jurnal IJESIT, 2014.
Sugiharto, dkk. 2016. Pengaruh Letak Magnet Terhadap
Konsumsi Bahan Bakar dan Emisi Gas Buang Pada
Electronic Fuel Injection Pada Sepeda Motor.
Malang: Universitas Widyagama Malang.
EIC 2018 - The 7th Engineering International Conference (EIC), Engineering International Conference on Education, Concept and
Application on Green Technology
20
