
Occurrence of Pathogenic Bacteria in Blood Cockles,
Anadara granosa
Nurin Syakirin Jantan, Zunita Zakaria, Saleha Abdul Aziz and Fuad Matori
Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
Keywords: Cockles, Pathogenic Bacteria, Antibiotic Susceptibility Test, Multidrug Resistance.
Abstract: Anadara granosa (blood cockles), also known as kerang is a very popular seafood in Malaysia and South
East Asia. In 2015, there has been a drastic reduction in the harvest and one of the main reasons is due to
the deteriorating water quality in the cockles' breeding environment. Thus, these cockles are exposed and at
high risk of being contaminated by pathogenic microorganism because they are filter-feeder organism. Most
of the researches on Anadara granosa have focused on only few selected organisms. The overall
microbiological assessment of the cockles is lacking therefore this study aimed to determine the types of
pathogenic bacteria in blood cockles, Anadara granosa and their antibiotic susceptibility pattern. Thirty
pooled sample of Anadara granosa were purchased from 15 wet markets and supermarkets within Klang
Valley. All samples were subjected to isolation and identification using standard conventional method. A
total of 85 isolates were successfully isolated and all were gram negative bacteria. Antibiotic susceptibility
test was performed for the different types of bacteria obtained. All isolates were found to be resistant to
Ampicilin (10 μg) and were sensitive to Trimethoprim/sulfamethoxazole (25μg). In conclusion, this study
showed that cockles are exposed to highly pathogenic bacteria and there is presence of antibiotic resistance.
1 INTRODUCTION
Anadara granosa is shellfish members of the Class
Bivalvia, mollusks that enclosed between two shells
which are closed together and joined together with
elastic hinge ligament (Sauders Veterinary
Dictionary, 2nd Edition. Malaysia produced
100,000 tons of cockles for both local consumption
and export. The cockles are harvested from the
coastlines especially in Selangor, Perak and Johor.
They usually distributed at tidal mudflat area with
low oxygen content due to presence of hemoglobin
that have ability to retain high oxygen content.
Cockles are exposed and at high risk of
contaminated by multiple organism, for instance
bacterial, viral and toxin-producing dinoflagellates
because they are filter-feeder organism such as
phytoplankton, zooplankton, bacteria, viruses and
inorganic materials (Burkhardt & Calci 2000;
Rippey, 1994). Consumption of cockles that is
harvested from contaminated area can cause illness
to human.
According to previous studies, it reveals that
aquatic environment is a reservoir for antibiotic
resistance due to frequent usage of antimicrobial and
antibiotic contamination (Samuel et al. 2016; Huang
et al. 2001). Thus, presence of pathogenic bacteria
together with multiple antibiotic resistances found in
aquaculture product will become a threat to public
health. This study was conducted to determine the
types of pathogenic bacteria in blood cockles and
determine the antibiotic resistance of selected
isolated bacteria.
2 MATERIALS AND METHODS
2.1 Sample and Data Collection
Thirty samples of Anadara granosa were purchased
from 15 wet markets and supermarkets. The shells
were rinsed by 70% alcohol and the meat was
removed aseptically. Three grams of the sample was
homogenized with 30ml of peptone water by
stomacher for 2minutes.
268
Jantan, N., Zakaria, Z., Aziz, S. and Matori, F.
Occurrence of Pathogenic Bacteria in Blood Cockles, Anadara granosa.
DOI: 10.5220/0008888602680272
In Proceedings of the 7th International Conference on Multidisciplinary Research (ICMR 2018) - , pages 268-272
ISBN: 978-989-758-437-4
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2.2 Methods
2.2.1 Isolation of Vibrio sp
The shell of Anadara granosa was cleaned using
70% alcohol. The meat was removed from the shells
and into 3g portions. The samples were then
homogenized with 30ml of alkaline peptone water
(Oxoid CM1028B) using stomacher for 2 minutes.
The homogenized sample was then incubated at 30
○
C for 24 hours under aerobic condition. After the
enrichment process, a lapful of the enriched sample
was streaked onto TCBS (CONDA). The plates
were incubated at 30
○
C for 24 hours under aerobic
condition.
2.2.2 Isolation of Bacteria Other than Vibrio
sp
The shell of Anadara granosa was cleaned using
70% alcohol. The meat was removed from the shells
and into 3g portions. The samples were then
homogenized with 30ml of peptone water (Oxoid
CM009) using stomacher for 2 minutes. After
homogenization, a lapful of each homogenized
sample was streaked onto Blood agar (Oxoid
CM0055) and MacConkey Agar (Oxoid CM1169).
The plates were incubated at 30
○
C for 24 hours
under aerobic condition.
2.2.3 Identification of Vibrio sp
Presumptive Vibrio sp exhibiting green and yellow
colonies on TCBS agar were selected and Gram
stained. These colonies were sub-cultured into
Triptych Soy (TSA) agar (BD #221283) in order to
obtain pure culture. The cultures were subjected to
series of biochemical test including oxidase test,
ability to growth at different NaCl concentration,
Voges–Proskauer test (VP), Lysine Decarboxylase
(LDC) and Ortho-Nitrophenyl-β-Galactoside
(ONPG) or species identification.
2.2.4 Identification of Bacteria Other than
Vibrio sp
Each different type of colony was picked and sub
cultured into Blood agar in order to obtain pure
colonies for identification. Gram staining
(Appendix A) was performed and series of
biochemical tests for gram negative and gram
positive were carried out. Biochemical tests include
blood broth, 6.5%NaCl, bile, lactose, sorbitol and
trehalose for Gram positive bacteria. Gram negative
bacteria were subjected to triple sugar iron (TSI),
Sulphide-Indole-Motility (SIM), urea and citrate 2.1
Isolation and identification.
2.2.5 Antibiotic Sensitivity Test
Susceptibility of the obtained bacteria to selected
antibiotics was tested on Mueller Hinton agar (MHA)
plates by the disc diffusion method according to
Bauer et al. (1966). One colony from pure culture was
emulsified in sterile saline solution until the turbidity
was match with standard 0.5 MacFarland solutions. A
sterile swab was dipped into the bacterial suspension
and then streaked over the entire surface of Mueller-
Hinton agar and Blood agar. Six antibiotic discs
Oxoid, were aseptically placed on the swabbed plates.
The antibiotics discs used include ampicillin (10μg),
erythromycin (15μg), tetracycline (30μg),
enrofloxacin (5μg), gentamicin (10μg), trimethoprim
and sulfamethoxazole (25μg), Anti biotic disc used in
this study were antibiotics that commonly used in
aquaculture as well as in human medicine. The plates
were incubated at 30°C for 24 h and the clear zone
formed around the discs was measured by using
caliper. The growth inhibition zone was compared
with zone-size interpretative table as in (CLSI, 2010).
3 RESULTS
The overall isolated bacteria in 30 different wet
markets and supermarkets around Klang Valley
revealed that a total of 85 isolates were successfully
isolated and representing 13 different types of
bacteria species (Figure 1). The study showed that
Aeromonas spp (23%) was the most frequently
isolated bacteria from cockles followed by Proteus
mirabilis (20%), Vibrio alginolyticus (15%), Vibrio
parahaemolyticus (6%), Photobacterium damsel
(6%), Vibrio cholera (5%), Chromobacterium sp.
(5%), Proteus vulgaris (2%), Salmonella spp (2%),
Klebsiella pneumoniae (2%), Plesiomonas
shigelloides (2%) and E.coli (1%).
Antibiotic susceptibility of the isolates was
performed using 6 antibiotics ranging from broad-
spectrum antibiotics and narrow spectrum. All of
the isolates showed resistance to at least one
antibiotic. Three isolates were multidrug resistance
as they are resistant to more than three types of
antibiotics from different classes. The bacteria
isolates showed the highest percentages of resistance
towards ampicillin (68%), followed by erythromycin
(37%), tetracycline (21%), enrofloxacin (16%),
gentamicin (11%) and trimethoprim/
sulfamethoxazole (11%). Isolates showed most
Occurrence of Pathogenic Bacteria in Blood Cockles, Anadara granosa
269
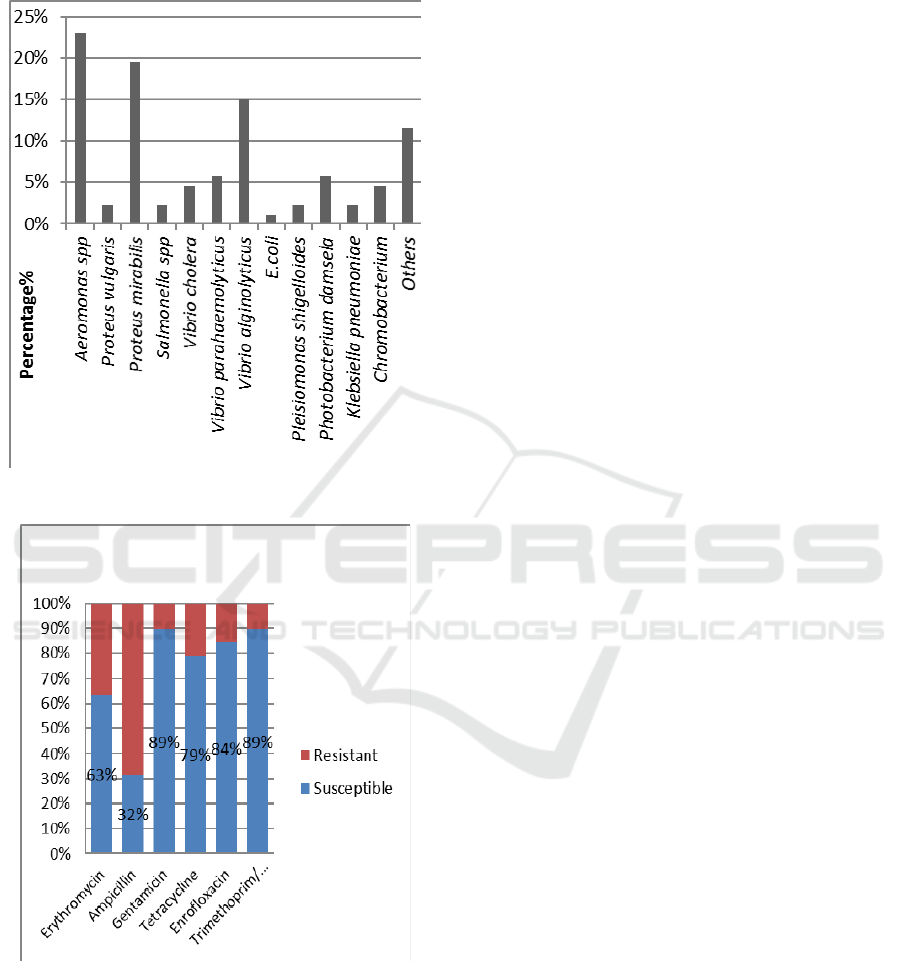
resistant towards ampicillin and most were sensitive
to trimethoprim/ sulfamethoxazole (Figure 2).
Figure 1: Types of bacteria isolated from blood cockles.
Figure 2: Percentage of resistant bacteria against different
antibiotics.
4 DISCUSSION
All isolated bacteria consisted of gram negative
bacteria. This concurs with the results obtained by
Santos et al. (2010), which stated that majority of
bacteria within marine environment are gram
negative bacteria. To date, very limited studies have
been carried out on the microbiology assessment and
antibiotic resistance in bivalves in Malaysia. One
of those studies revealed that 93% of the isolated
bacteria were gram negative bacteria (Ahmad,
2014).
Bacteria obtained from this study can be
categorized into 2 family groups which are
Vibrionacea and Enterobacteriae. These two groups
can be easily differentiated by oxidase test for which
the Vibrionaceae will give positive result. These two
groups of family are mostly pathogens that usually
cause gastroenteritis in human. Aeromonas is the
most abundant isolated bacteria in this study.
Gastrointestinal infections caused by Aeromonads
are mostly self-limiting, and antibiotic therapy is
required only in chronic cases of immunosuppressed
hosts (Igbinosa, 2012). The least isolated bacteria
were from family of Enterobacteriaceae which are
E.coli (1%), Salmonella spp (2%), Klebsiella
pneumonia (2%), Plesiomonas shigelloides (2%),
Proteus vulgaris (2%) and Proteus mirabilis (20%).
Increase in bacterial contamination at beaches along
many coastlines usually occurred during heavy
rainfall or rainy season (Gregory, 2009). In this
study, sampling was done on the dry season in the
West Coast of Peninsular Malaysia, therefore it is
predicted more contaminants might be seen if it is
the rainy season.
According to Letchumanan (2014), Malaysia is
one of the Asian countries that often suffer food
borne outbreaks mainly caused by Vibrio sp. Other
countries include Japan, India, China, Taiwan and
Korea. Three members of the Vibrio genus were
isolated in this study were Vibrio alginolyticus,
Vibrio cholera and Vibrio parahaemolyticus. This
finding is in agreement with the studies of Ahmad
(2014) and Thompson (2014) which stated that
Vibrio sp is the most dominant genus present in
cockles. Vibrio sp have unique ability whereby they
are hardy organism as they able to withstand harsh
environment. They can be found in a wide range of
environment; from estuaries, coastal, marine water
and even sediment. In a previous study conducted
by Wan and Nor (2004) on bacterial quality of some
shellfish revealed that Vibrio spp. are commonly
isolated from cockles compared to other shellfish.
Some limitation on isolation and identification of
bacterial pathogens by using conventional methods
is lack of sensitivity as stated in Law et.al, 2014 and
this may lead to false negative e results. In addition,
marine organisms might occur in a state which is
viable but non-cultural. In this study approximately
ICMR 2018 - International Conference on Multidisciplinary Research
270

11% of bacteria cultures were not able to be
identified, and this may be attributed to the
limitations of the conventional identification system
employed.
Multidrug resistance is defined as bacteria that
are resistant to 3 or more antimicrobial classes. In
this study, 3 isolates namely Aeromonas sp., Vibrio
parahaemolyticus and Klebsiella pneumoniae were
found to be multidrug resistant (MDR). Based on
one study done by Ghaderpour et al. (2015),
emergence of resistance of bacteria is associated
with anthropogenic pollution in Matang estruary,
Kuala Sepetang. This estuary is one of the major
cockles producing area. This estuary was
contaminated with untreated silages that contain
organic materials, household chemicals and
pathogens therefore contaminated the cockles
breeding environment. The presence of these MDR
is alarming as infections caused by these resistant
organisms are difficult to treat.
5 CONCLUSIONS
This study suggests that Anadara granosa have poor
microbiological quality and harbor various
pathogenic bacteria. Trimetoprim/ sulfamethoxazole
and gentamicin are most effective in eliminating
bacteria in cockles. A number of the pathogenic
bacteria obtained namely Aeromonas sp., Vibrio
parahaemolyticus and Klebsiella pneumoniae
exhibited multidrug resistant trait.
REFERENCES
Ahmad, F., Ismail, N., Jaafar, H., Nordin, W. N., Telipot,
M., Pinang, P., & Sepetang, K., 2007. Bacteriological
Comparison Of Cockles From Three Producing Areas
In Peninsular Malaysia, 18(2), 35–44.
Akinbowale, O. L., Peng, H., & Barton, M. D., 2006.
Antimicrobial resistance in bacteria isolated from
aquaculture sources in Australia. Journal of Applied
Microbiology, 100(5), 1103–1113.
https://doi.org/10.1111/j.1365-2672.2006.02812.
Asmat, A., Mehat, D. N., Rahimi, H., & Gires, U., 2014.
Population density and antibiotic resistant of bacteria
from bivalve (Perna viridis and Anadara granosa).
Sains Malaysiana, 43(4), 543–550.
Al-Othrubi S.M.Y., Kqueen, C.Y., Mirhosseini, C.Y.,
Hadi, Y.A., Radu, S., 2014. Antibiotic Resistance of
Vibrio parahaemolyticus Isolated from Cockles and
Shrimp Sea Food Marketed in Selangor, Malaysia.
Clin Microbial 3: 148. doi:10.4172/2327-
5073.1000148
Burkhardt W, Calci K.R., 2000. Selective accumulation
may account for shellfish-associated viral illness. Appl
Environ Microbiol. 66:1375–1378. doi:
10.1128/AEM.66.4.1375-1378.2000.
Castro, D., Pujalte, M.J., Lopez-Cortes, L., Garay, E. &
Borrego, J.J. 2002. Vibrios isolated from the cultured
manila clam (Ruditapes philippinarum): Numerical
taxonomy and antibacterial activities. Journal of
Applied Microbiology 93: 438-447.
Ghaderpour, A., Ho, W. S., Chew, L.-L., Bong, C. W.,
Chong, V. C., Thong, K.-L., & Chai, L. C. (2015).
Diverse and abundant multi-drug resistant E. coli in
Matang mangrove estuaries, Malaysia. Frontiers in
Microbiology, 6, 977.
http://doi.org/10.3389/fmicb.2015.00977
Hassan R., Kanakaraju D., 2013 Razor clams (Class
Bivalvia) of Kuala Selangor, Malaysia: morphology,
genetic diversity and heavy metal concentration.
Borneo Journal of Resource Science and Technology
2(2):19–27.
Huang, C.H., Renew, J.E., Smeby, K.L., Pinkerston, K. &
Sedlak, D.L. 2001. Assessment of potential antibiotic
contaminants in water and preliminary occurrence
analysis. Water Resour. Update 120: 30-40.
Heuer, O. E., Kruse, H., Grave, K., Collignon, P.,
Karunasagar, I., & Angule, F. J., 2009. Human Health
Consequences of Use of Antimicrobial Agents in
Aquaculture. Food Safety, 1248-1253.
Iwamoto, M., Ayers, T., Mahon, B. E., & Swerdlow, D.
L., 2010. Epidemiology of seafood-associated
infections in the United States. Clinical Microbiology
Reviews, 23(2), 399–411.
https://doi.org/10.1128/CMR.00059-09
Jayasinghe, L., Ahmed, N., & Kariyawasam, U., 2005.
The Isolation and Identification of. Wayamba
University of Sri Lanka, 1–6.
Law, J. W, Ab Mutalib, N, Chan, K, & Lee, L., 2014.
Rapid methods for the detection of foodborne bacterial
pathogens: principles, applications, advantages and
limitations. Frontiers in Microbiology, 5, 770.
http://doi.org/10.3389/fmicb.2014.00770
Letchumanan, V., Chan, K.-G., & Lee, L.-H., 2014. Vibrio
parahaemolyticus: a review on the pathogenesis,
prevalence, and advance molecular identification
techniques. Frontiers in Microbiology, 5, 705.
http://doi.org/10.3389/fmicb.2014.00705
Liong, P. C., Hanafi, H. B., Merican, Z. O., Nagaraj, G.,
1988. Aquaculture development in Malaysia. In J. V.
Juario & L. V. Benitez (Eds.), Perspectives in
Aquaculture Development in Southeast Asia and
Japan: Contributions of the SEAFDEC Aquaculture
Department. Proceedings of the Seminar on
Aquaculture Development in Southeast Asia, 8-12
September 1987, Iloilo City, Philippines. (pp. 73-90).
Tigbauan, Iloilo, Philippines: SEAFDEC, Aquaculture
Department.
Ole E. Heuer, Hilde Kruse, Kari Grave, P. Collignon,
Iddya Karunasagar, Frederick J. Angulo; Human
Health Consequences of Use of Antimicrobial Agents
Occurrence of Pathogenic Bacteria in Blood Cockles, Anadara granosa
271

in Aquaculture. ClinInfect Dis 2009; 49 (8): 1248-
1253. doi: 10.1086/605667
Potasman I, Paz A and Odeh M., 2002. Infectious
outbreaks associated with bivalve shellfish
consumption: A worldwide perspective. Clin. Infect.
Dis. 35: 921–928.
Rippey, S. R., 1994. Infectious diseases associated with
molluscan shellfish consumption. Clinical
Microbiology Reviews, 7(4), 419–425.
Sartori, André F., 2015. Anadara granosa (Linnaeus,
1758). In: MolluscaBase., 2015. Accessed through:
World Register of Marine Species at
http://www.marinespecies.org/aphia.php?p=taxdetails
&id=715138 on 2017-02-01
Thompson, F.L., Iida, T. & Swings, J., 2004. Biodiversity
of Vibrios. Microbiology and Molecular Biology
Reviews 68: 403-431.
Wan Norhana N and Nor Ainy M., 2004. Bacteriological
quality of some molluscan shellfish from growing
waters of Peninsular Malaysia. Malaysia Fisheries J.
3(1): 27–38.
Yap, C. K, Razeff S. M. R, Edward F. B, and Tan S. G,
“Heavy metal concentrations (Cu, Fe, Ni and Zn) in
the clam, Glauconome virens, collected from the
northern intertidal areas of Peninsular Malaysia,”
Malaysian Applied Biology Journal, vol. 38, no. 1, pp.
29–35, 2009.
ICMR 2018 - International Conference on Multidisciplinary Research
272
