
Density Functional Theory Studies on Guanine and Cytosine
W. N. Zaharim
1*
, S. Sulaiman
1,2
, S. N. Abu Bakar
1
, N. E. Ismail
1
, H. Rozak
1,3
and I. Watanabe
1,3
1
Computational Chemistry and Physics Laboratory, School of Distance Education, Universiti Sains Malaysia, Pulau
Pinang 11800, Malaysia.
2
USM-RIKEN International Center for Ageing Science, School of Distance Education, Universiti Sains Malaysia, Pulau
Pinang 11800, Malaysia.
3
Advanced Meson Science Laboratory, RIKEN Nishina Center, Wako, Saitama 351-0198, Japan
nureliana_ismail@yahoo.com, harisonrozak7@gmail.com
Keywords: DNA, electron transport, Density Functional Theory, electronic structure.
Abstract: DFT cluster method was employed to investigate the electronic structures of guanine and cytosine in the
form of nucleobase and nucleotide. All calculations were performed at the B3LYP/6-311++G (d,p) level.
From the computational study, the presence of methyl group or sugar phosphate group to the nucleic acid
bases has a direct effect on the structure of the system. The planar structure of the nitrogenous base is
maintained after geometry optimization procedure. No significant difference was found in the charge
distribution in nucleobase and nucleotide for both guanine and cytosine. The ionization energy for guanine
is found to be lower than that for cytosine. The HOMO-LUMO gap is lower for both guanine and cytosine
in the nucleobase form. The calculated dipole moment shows that guanine is more polarized than cytosine.
1 INTRODUCTION
Deoxyribonucleic acid (DNA) is a versatile
molecule that stores genetic information and consists
of two polynucleotide chains twisted around each
other in the form of a double helix. DNA is formed
using sequences of four nitrogenous bases. These
four bases are guanine (G), adenine (A), cytosine
(C) and thymine (T), and they can be divided into
two groups. Guanine and adenine belong to the
purine group which is considered as a good electron
donor. Cytosine and thymine on the other hand
belong to the pyrimidine group which is poor
electron donor as compared to the purine group.
Purines and pyrimidines are considered as aromatic
compounds and are electron rich in nature (Garrett
and Grisham, 2002). The molecular formulas for
adenine, guanine, cytosine and thymine bases are
C
5
H
5
N
5
, C
5
H
5
N
5
O, C
4
H
5
N
3
O, and C
5
H
6
N
2
O
2
respectively (Kim et al. 2015). In double strand
DNA, the four bases are arranged in a pair by
forming hydrogen bond between the bases. Adenine
on one strand will pair up with thymine base on the
other strand, while guanine pair up with cytosine.
These nitrogenous bases will be ordered in some
ways to provide the necessary information to make
proteins for building and maintaining an organism.
All four nitrogenous bases have different electronic
structure and chemical structure. The molecular
orbital and electron distribution for all nitrogenous
bases are different from one another.
DNA bases are considered as an organic
molecular compound that has the ability to transport
electron through (Chakraborty, 2007). In general,
electron transport occurs in many important
biological processes such as the storage and
consumption of energy, enzyme response, and DNA
UV damage repair (Klotsa et al. 2005). Electron
transport along the DNA molecule and the potential
application of DNA molecule has caught the
attention of many chemists and physicists. DNA is a
biological molecule that has potential in fabrication
and construction of electronic nanodevices due to
their electronic properties (Cai et al. 2000 and Kaur
et al. 2011). Studies using varieties of experimental
approaches show that DNA is an effective medium
for charge migration (Apalkov et al. 2007). To study
the electron transport or charge migration through
DNA, the electronic structure of DNA bases need to
be known.
There are varieties of experimental techniques
that were used to determine the structure of DNA
Zaharim, W., Sulaiman, S., Abu Bakar, S., Ismail, N., Rozak, H. and Watanabe, I.
Density Functional Theory Studies on Guanine and Cytosine.
DOI: 10.5220/0008887000850091
In Proceedings of the 7th International Conference on Multidisciplinary Research (ICMR 2018) - , pages 85-91
ISBN: 978-989-758-437-4
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
85
nitrogenous bases such as NMR spectroscopy, X-ray
crystallography, and electron microscopy. The
experimental results are often compared with
theoretical results. Several research groups have
carried out theoretical study by using isolated bases
(hydrogen replace sugar phosphate) (Kilina et al.
2007) or methyl group (Mahanto et al. 2008) to
replace the sugar phosphate backbone. The use of
methyl group to replace sugar phosphate group that
is attached to the bases can be a justifiable
approximation as it can mimic the effect of sugar
phosphate group through hyperconjugation effects
(Mahato et al. 2008). However, methyl group is an
electron donating group that could increase electron
donating properties of the molecule (Pullman and
Pullman, 1958). Mahanto et al. also concluded that
that sugar phosphate group needs to be taken into
consideration when designing a calculation model
(Mahanto et al. 2008). The question on the effect of
methyl group and sugar phosphate group to the
electronic structure of the nitrogenous base is
therefore the motivation of this study.
In this paper, we report the results of our
computational investigation using DFT cluster
method to study the electronic structures of one of
the complementary base pairs which are guanine and
cytosine. The goal of this study is to investigate the
electronic structure of guanine and cytosine in two
different forms.
2 METHODOLOGY
The structural data for all four structures studied in
this investigation guanine nucleobase, guanine
nucleotide, cytosine nucleobase and cytosine
nucleotide were obtained from PubChem database
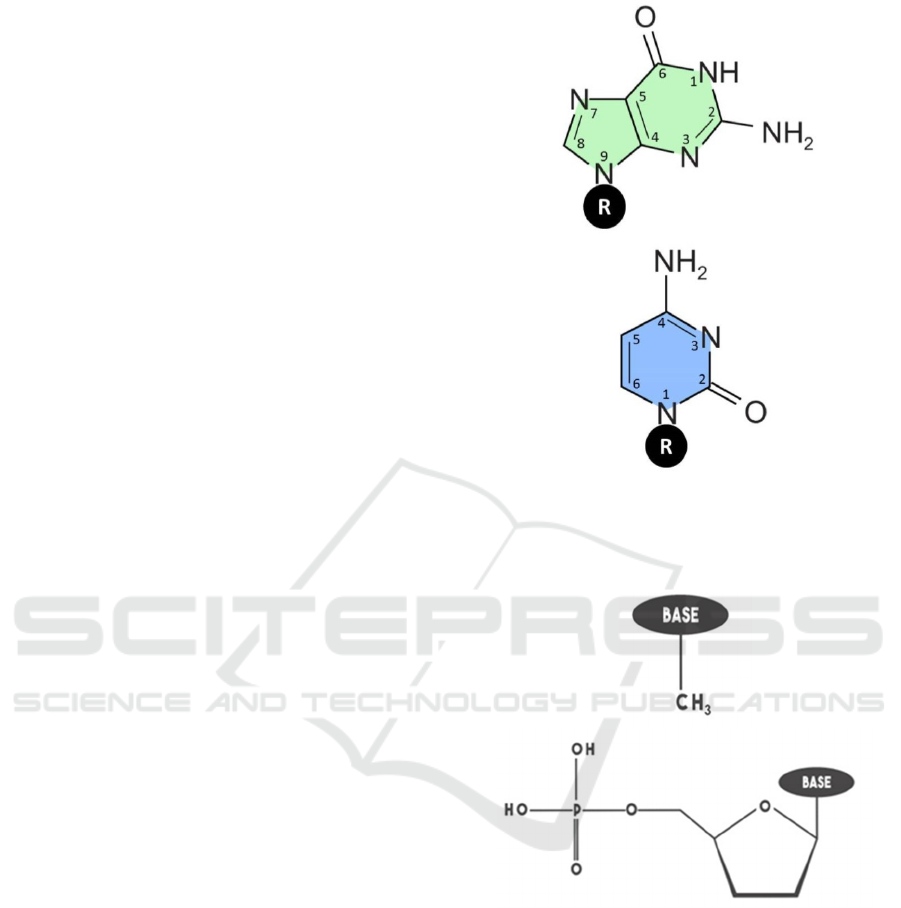
(Kim et al. 2015). Figure 1 represents guanine and
cytosine structures. The atoms numbering follows
Sinden et al. numbering scheme (Sinden et al. 1998).
The difference between nucleobase and
nucleotide structures is that for the former a methyl
group is attached at the R position, whereas for the
later a sugar phosphate group is attached as shown in
Figure 1. The structures of the methyl group and
sugar phosphate groups are shown in Figure 2. A
methyl group belongs to an organic family called an
alkyl group that contains one carbon atom
surrounded by three hydrogen atoms. Sugar
phosphate group is an important structural
component that forms the backbone of nucleic acids
such as DNA and RNA. A sugar phosphate group
consists of deoxyribose sugar that attaches to a
phosphate group.
Figure 1: DNA bases structures and numbering. (a)
guanine and (b) cytosine.
Figure 2: Methyl group and sugar phosphate structures. (a)
methyl group and (b) sugar phosphate.
DFT cluster method was applied to study the
electronic structures of all four structures. DFT
method is an alternative ab initio method that is
more efficient in considering correlation energy.
Hybrid functional is the most popular functional
used in DFT computations. One common functional
is B3LYP. Hybrid functional is effective functional
for organic, biochemical and large systems without
requiring an excessive amount of computing time,
memory and disk space (Rengifo and Murillo,
2012).
a)
b)
a)
b)
ICMR 2018 - International Conference on Multidisciplinary Research
86
The Cluster Method was employed
(Sulaiman et al. 2015) and the DFT quantum
mechanical procedure at B3LYP/6-311++G (d,p)
level (Izzati et al. 2011) was applied to investigate
the electronic structures. The chosen basis set
contains a polarization function which is important
to allow the distortion of the atomic orbitals in a
molecular environment. The 6-311++G basis set
used in our investigation has p-type function added
to hydrogen atom and d-type function added to all
other atoms. The diffuse function which is also
included in the 6-311++G (d,p) basis sets is to
allows the electron to move further away from the
nucleus in the ground state (diffuse functions were
added to all atoms). The extended basis set is needed
to produce a reliable result of the electronic structure
and total energy (Rengifo and Murillo, 2012). The
converged molecular orbitals were then used to
examine the electronic structures of the systems.
Gaussian 16 (G16) computational software
package installed at the RIKEN Hokusai Great
Wave Supercomputing facility was used to perform
the calculations. Gaussian 16 is the latest in the
Gaussian series of programs and has the capabilities
for electronic structure modelling (Frisch et al.
2016). There are a few types of calculation that can
be done by using this computational software such
as single point energy, geometry optimization,
Hartee-Fock and others.
Two types of calculations were performed in this
study, single point energy calculation and geometry
optimization calculation. Single point energy
calculations were made to compute the electronic
structure of the system and provide information
about the molecule such as energy, wave function
and charge distribution. The calculation is performed
at a single fixed geometry. On the other hand,
geometry optimization calculations were conducted
to determine the geometry of the molecules that is
the most stable with respect to the total energy. All
four structures were optimized such that the
optimized geometries correspond to the systems
with minimum total energy.
3 RESULT AND DISCUSSION
Following the main motivation of our computational
investigation, a systematic study on the electronic
structures of guanine and cytosine that are attached
to a methyl group or a sugar phosphate group has
been performed using the methodology presented
above.
3.1 Geometrical Parameters
All optimized structures preserved the planar
geometrical shape of the nitrogenous bases after
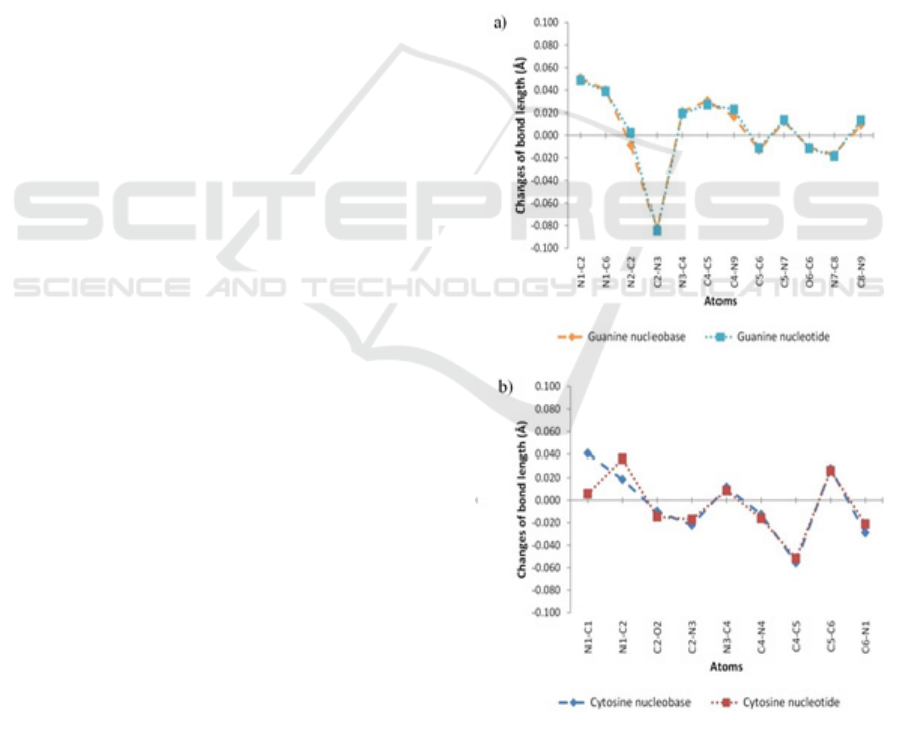
atomic relaxation. Figure 3 shows the changes in the
selected bond lengths of the optimized structure
relative to the initial structure, while Figure 4 shows
the changes in the selected bond angles. As can be
seen from Figure 3 and Figure 4, the trend of the
increases or decreases of the parameters are the
same for most atoms in nucleobases and nucleotides
form. The addition of methyl group or sugar
phosphate group results in similar effects to the bond
lengths and bond angles. The changes in the bond
lengths and bond angles do not result in any
significant modification to the planar shape of the
nitrogenous bases.
Figure 3: Bond length changes. (a) guanine and (b)
cytosine.
Density Functional Theory Studies on Guanine and Cytosine
87
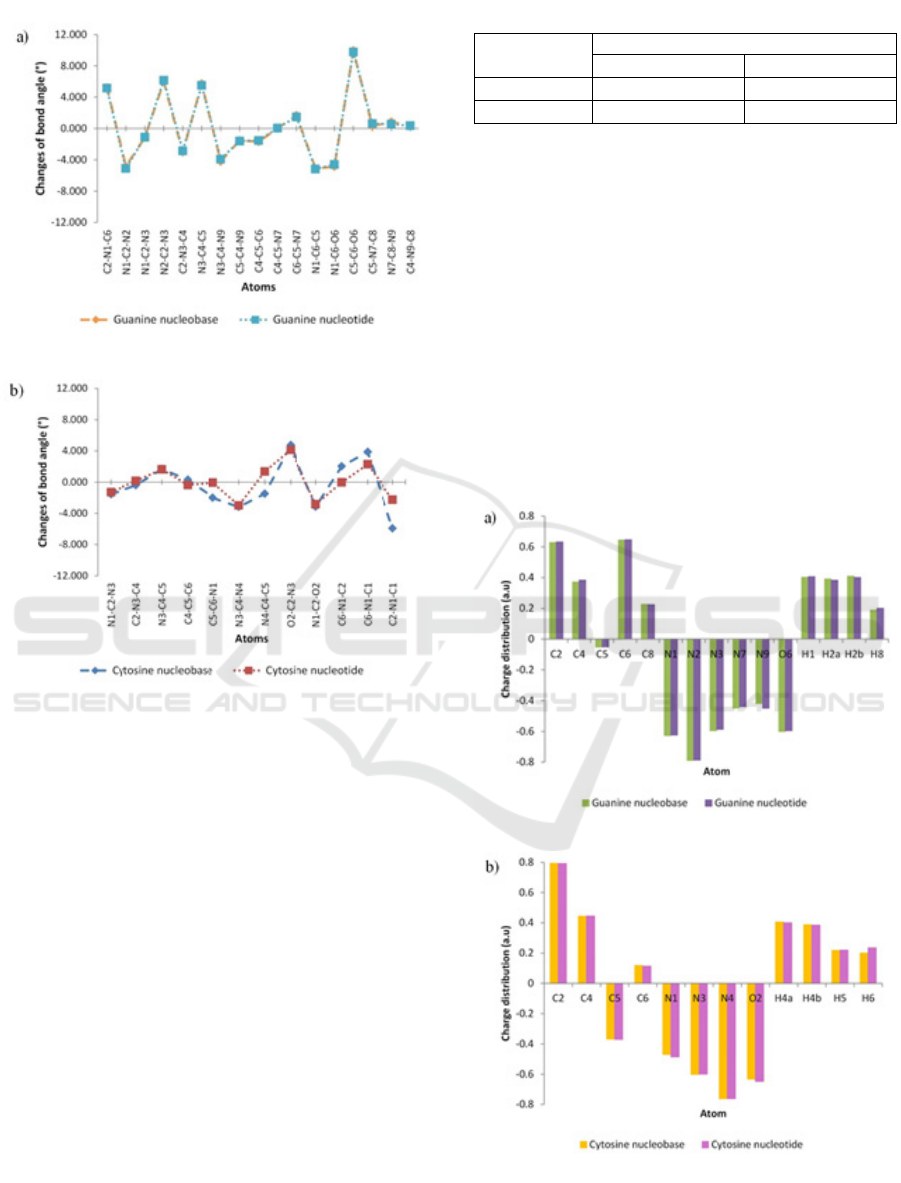
Figure 4: Bond angles change of optimized structure. (a)
guanine and (b) cytosine.
3.2 Total Energy
The total energy of each molecule after geometry
optimization procedure is given in Table 1. The total
energy presented is relative to total energy obtained
from single point energy calculation. This result
indicates that the optimized structure is more stable
because it has a lower energy. Nitrogenous base with
attached sugar phosphate group experiences large
differences in the energy after geometry
optimization process. It is therefore very important
to perform geometry optimization procedure before
attempting to obtain the electronic structure of the
system studied.
Table 1: Total energy of guanine and cytosine.
Nitrogenous
base
Total energy (eV)
Nucleobase Nucleotide
Guanine - 0.584 - 1.029
Cytosine - 0.137 - 0.634
3.3 Charge Distribution
Mulliken population analysis and natural population
analysis are two population analyses that can be
used to determine charge distribution in a molecule.
Mulliken population analysis is the most common
population analysis that has been used due to its
simplicity (Šponer et al. 2001). In this study, we did
not use Mulliken population analysis to determine
the effective charge on the atoms because of the
result is dependent of the method and basis set
(Matczak, 2016). The charge distribution presented
in this study is based on the natural population
analysis.
Figure 5: Atomic charge distribution. (a) guanine and (b)
cytosine.
ICMR 2018 - International Conference on Multidisciplinary Research
88
The determination of charge distribution is
crucial in the study of the electronic structure of the
system. Atomic charge distribution can be used to
study the charge transfer in a chemical reaction.
Figure 5 represents the charge distribution value of
guanine and cytosine optimized structures. The
existence of methyl group and sugar phosphate
group does not give significant effect to the charge
distribution around the nitrogenous bases ring.
3.4 Ionization Energy
The calculated ionization energies for the optimized
structures are summarized in Table 2. From the
result, it can be seen that the ionization energy
increases by 0.364 eV when the methyl group is
replaced by a sugar phosphate group. Thus, it
requires more energy to remove an electron from the
latter molecule. In the case of cytosine, the
difference in the ionization energy between
nucleobase and nucleotide is not only relatively
small, but decreases, which is opposite in effect as
compared to the case of guanine.
From our calculation, guanine in nucleobase and
nucleotides configurations has lower ionization
energies as compared to cytosine. Our results are in
agreement with the statement of Senthilkumar et al.
(Senthilkumar et al. 2003). Considering that guanine
has a lower ionization energy, guanine base becomes
the main target for oxidation to occur (Seidel et al.
1996). From biological perspective, oxidation of
nitrogenous base promotes oxidative damage.
Table 2: Ionization energy of guanine and cytosine.
Nitrogenous
base
Ionization energy (eV)
Nucleobase Nucleotide
Guanine 5.849 6.258
Cytosine 6.462 6.459
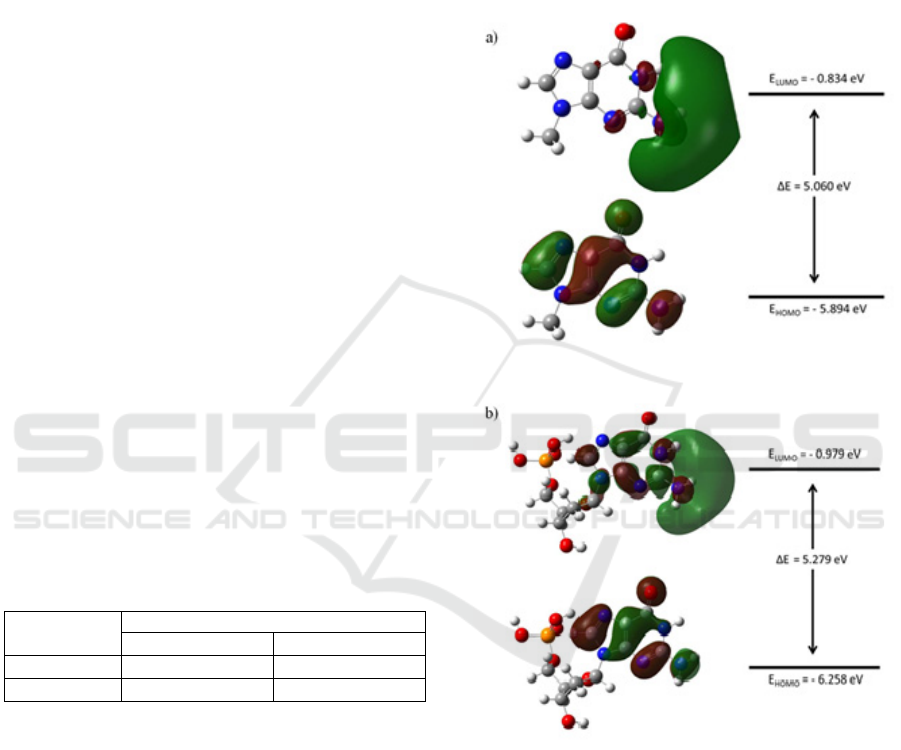
3.5 Frontier Molecular Orbital
Analysis
The transfer of charge through the molecule is
affected by the electronic structure (Padmaja et al.
2009). In particular, the difference in the energy of
highest occupied molecular orbital (HOMO) and
lowest occupied molecular orbital (LUMO) is an
important parameter that is considered to study and
understand the possibilities of charge migration
through DNA. The surface plot of the calculated
HOMO and LUMO, and the corresponding energy
level diagram are shown in Figure 6 and to Figure 7.
The molecular orbital is presented on the surface
of equal amplitude of 0.020. From this plot, the
positive and negative sign wave function is indicated
by the red and green colour respectively. The sign of
the wave function on the nitrogenous base ring in
nucleobase and nucleotide configuration is opposite
from each other. It is clear that methyl group and
sugar phosphate groups have an impact on the
attributes of the molecular orbitals.
Figure 6: Molecular orbital surface plot and energy level
diagram of guanine. (a) guanine nucleobase and (b)
guanine nucleotide.
Density Functional Theory Studies on Guanine and Cytosine
89
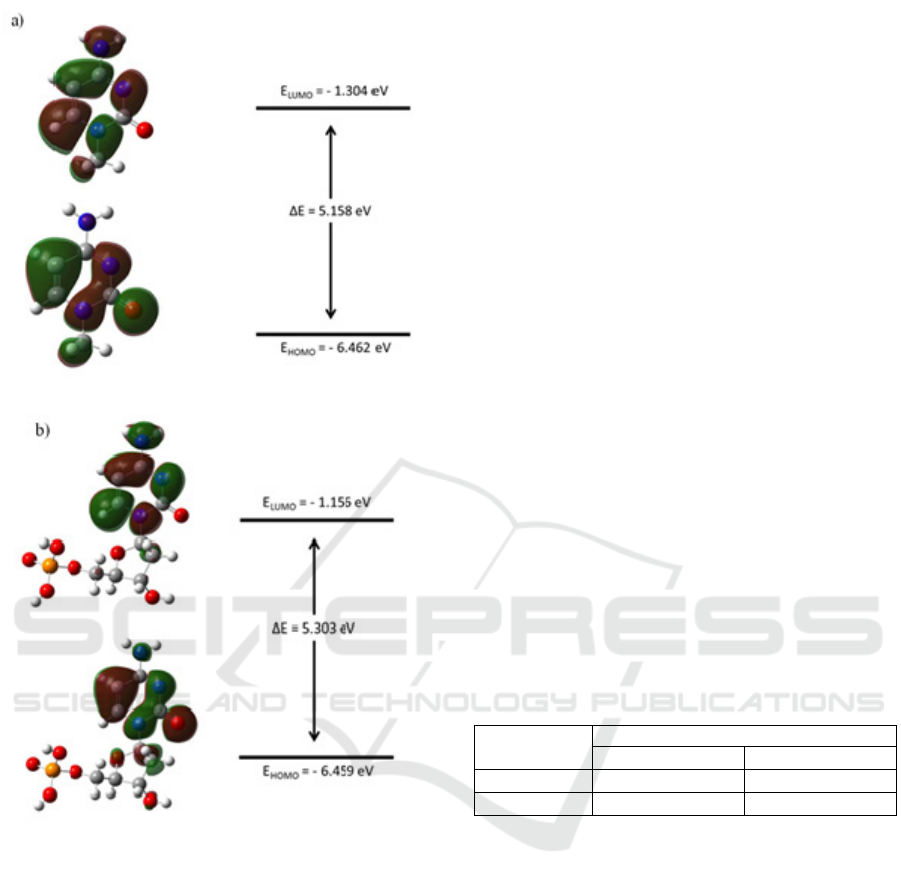
Figure 7: Molecular orbital surface plot and energy level
diagram of cytosine. (a) cytosine nucleobase and (b)
cytosine nucleotide.
From the molecular orbital energy diagram, it
can be seen that all nucleobases and nucleotides
have a HOMO-LUMO gap of more than 5 eV. The
value of HOMO-LUMO gap for guanine
nucleobase, guanine nucleotide, cytosine nucleobase
and cytosine nucleotide are 5% lower, 9% lower, 1%
lower and 2% higher than Kilina et al. findings
respectively (Kilina et al. 2007). The sequence and
magnitude of the HOMO-LUMO gap for all
structure are guanine nucleobase < cytosine
nucleobase < guanine nucleotide < cytosine
nucleotide. Nucleobases structures have a lower
HOMO-LUMO gap. This is more likely due to the
presence of methyl group. Methyl group is an
electron donating group and could decrease the
HOMO-LUMO gap (Pullman and Pullman, 1958).
Structure with sugar phosphate group attached to the
nitrogenous base has a larger HOMO-LUMO gap.
Hence, it is clear that inclusion of sugar phosphate
group has an impact to the HOMO-LUMO gap. The
changes in the HOMO-LUMO gap could affect the
possibilities of charge transport through DNA.
3.6 Dipole Moment
The measurement of dipole moment is important in
differentiating polar and non-polar molecules.
Dipole moment is basically the measure of net
polarity in a molecule. Polar molecule has an uneven
charge distribution across the entire molecule.
Moreover, the polarity is determined by the
distribution of donor and acceptor functional group
around the base.
Guanine and cytosine are known as polar
molecules (Kilina et al. 2007). Table 3 summarizes
the values of dipole moment of guanine and cytosine
calculated using the optimized structures. From the
calculated dipole moment, the most polar base is
guanine. Cytosine has a lower dipole moment. For
guanine, the dipole moment of the nucleotide is
slightly larger than that for the nucleobase.
However, the trend is opposite for the case of
cytosine.
Table 3: Dipole moment of guanine and cytosine.
Nitrogenous
base
Dipole moment (D)
Nucleobase Nucleotide
Guanine 7.400 7.413
Cytosine 6.369 5.322
4 CONCLUSION
From our computational study, the presence of
methyl group or sugar phosphate group to the
nucleic acid bases has a direct effect on the structure
of the system. Further computational investigation
should be performed on adenine and thymine. A
comparison between all four bases is needed so that
we can have a better understanding on DNA
electronic structure and properties.
The computational method that was used in this
study can be employed for more complex oligomer
system. This study would be extended using bigger
cluster size with more bases so that the effects of
neighbouring bases can be included and studied.
ICMR 2018 - International Conference on Multidisciplinary Research
90

ACKNOWLEDGEMENTS
This research was supported by Universiti Sains
Malaysia through Research University grant (Grant
No: 1001/PJJAUH/870037. We would like to
acknowledge Hokusai Greatwave Supercomputing
facility (Project No. G18022) at RIKEN Advanced
Center for Computing and Communication. One of
us (W.N. Zaharim) would like to thank the Ministry
of Education, Malaysia for the award of MyBrain 15
fellowship.
REFERENCES
Apalkov, V., Wang, X. F., & Chakraborty, T., 2007.
Physics aspects of charge migration through DNA.
In Charge Migration in DNA (pp. 77-119). Springer,
Berlin, Heidelberg.
Cai, L., Tabata, H., & Kawai, T., 2000. Self-assembled
DNA networks and their electrical
conductivity. Applied Physics Letters, 77(19), 3105-
3106.
Chakraborty, T. (Ed.)., 2007. Charge migration in DNA:
perspectives from physics, chemistry, and biology.
Springer Science & Business Media.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G.
E., Robb, M. A., Cheeseman, J. R., . . . Fox, D. J.
(2016). Gaussian 16 Rev. B.01. Wallingford, CT.
Garrett, R. H., & Grisham, C. M., 2002. Principles of
biochemistry: with a human focus. Orlando, Florida:
Harcourt College Publishers.
Izzati, T., Sulaiman, S. and Ismail, M., 2011. DENSITY
FUNCTIONAL THEORY: HYBRID FUNCTIONAL
STUDY OF TETRAPHENYLTIN. Science
International, 23(4).
Kaur, D. K., Bharadwaj, L. M., Kaur, I., & Singh, M. L.
(2011). Tunneling effects in DNA bases Adenine and
Guanine. International Journal of Computer
Applications, 17(1).
Kilina, S., Tretiak, S., Yarotski, D. A., Zhu, J. X., Modine,
N., Taylor, A., & Balatsky, A. V., 2007. Electronic
properties of DNA base molecules adsorbed on a
metallic surface. The Journal of Physical Chemistry
C, 111(39), 14541-14551.
Kim, S., Thiessen, P. A., Bolton, E. E., Chen, J., Fu, G.,
Gindulyte, A., Han, L., He, S., Shoemaker. B.A. &
Wang, J., 2015. PubChem substance and compound
databases. Nucleic acids research, 44(D1), D1202-
D1213.
Klotsa, D., Römer, R. A., & Turner, M. S., 2005.
Electronic transport in DNA. Biophysical journal,
89(4), 2187-2198.
Mahato, D. N., Dubey, A., Pink, R. H., Scheicher, R. H.,
Badu, S. R., Nagamine, K., Torikai, E., Saha, H.P.,
Chow, L., Huang, M.B. & Das, T. P., 2008.
Theoretical investigation of nuclear quadrupole
interactions in DNA at first-principles level. In
HFI/NQI 2007 (pp. 601-606). Springer, Berlin,
Heidelberg.
Matczak, P., 2016. A test of various partial atomic charge
models for computations on diheteroaryl ketones and
thioketones. Computation, 4(1), 3.
Padmaja, L., Ravikumar, C., Sajan, D., Hubert Joe, I.,
Jayakumar, V. S., Pettit, G. R., & Faurskov Nielsen,
O., 2009. Density functional study on the structural
conformations and intramolecular charge transfer from
the vibrational spectra of the anticancer drug
combretastatinA2. Journal of Raman Spectroscopy:
An International Journal for Original Work in all
Aspects of Raman Spectroscopy, Including Higher
Order Processes, and also Brillouin and Rayleigh
Scattering
, 40(4), 419-428.
Pullman, B., & Pullman, A., 1958. Electron-donor and-
acceptor properties of biologically important purines,
pyrimidines, pteridines, flavins, and aromatic amino
acids. Proceedings of the National Academy of
Sciences, 44(12), 1197-1202.
Rengifo, E., & Murillo, G., 2012. DFT-based investigation
of the electronic structure of a double-stranded AC B-
DNA dim. Revista de Ciencias, 16, 117-122.
Seidel, C. A., Schulz, A., & Sauer, M. H., 1996.
Nucleobase-specific quenching of fluorescent dyes. 1.
Nucleobase one-electron redox potentials and their
correlation with static and dynamic quenching
efficiencies. The Journal of Physical
Chemistry, 100(13), 5541-5553.
Senthilkumar, K., Grozema, F. C., Guerra, C. F.,
Bickelhaupt, F. M., & Siebbeles, L. D. A., 2003.
Mapping the sites for selective oxidation of guanines
in DNA. Journal of the American Chemical Society,
125(45), 13658-13659.
Sinden, R. R., Pearson, C. E., Potaman, V. N., & Ussery,
D. W., 1998. DNA: structure and function. In
Advances in genome biology (Vol. 5, pp. 1-141). JAI.
Šponer, J., Leszczynski, J., & Hobza, P., 2001. Electronic
properties, hydrogen bonding, stacking, and cation
binding of DNA and RNA bases. Biopolymers:
Original Research on Biomolecules, 61(1), 3-31.
Sulaiman, S., Ahmad, S.N.A., Mohamed-Ibrahim, M.I.
and Watanabe, I., 2015. Electronic Structure of
Muonated Me4P [Pd (dmit) 2] 2. In Materials Science
Forum (Vol. 827, pp. 355-359). Trans Tech
Publications.
Density Functional Theory Studies on Guanine and Cytosine
91
