
Effect of Addition Silica Gel from Volcanic Ash of Sinabung
Mountains to Tensile Strength on Chitosan Composite Membrane
Maulida
1
, Lilis Sukeksi
1
, Mara Bangun Harahap
2
, Melva Ginting
1
, Herlinawati Wici
1
and Ghendis
Ekawati Ayu
1
1
Departement of Chemical Engineering, Faculty of Engineering, University of Sumatera Utara, Almamater Street,Medan,
20155, Indonesia
2
Departement of Physics, Faculty of Mathematic and Science, University of Negeri Medan, Medan Estate, North Sumatera
20222, Indonesia
Keywords: Silica gel, volcanic ash, chitosan, composite membrane
Abstract: Silica gel is a glassy grain with a very porous shape. Silica is made synthetically from sodium silicate. In
this study, volcanic ash can be used as silica, which is silica can be applied in the manufacture of composite
membranes as fillers because it can increase the conductivity of composite membranes. The method used
for making silica gel was extraction method and composite membrane made using phase inversion method
with the composition of chitosan and silica used are 2 grams of chitosan and variations of fillers 0.6, 0.9 and
1.2 grams of silica, and the stirring time is 8,12 and 16 hours. The characteristics and analysis carried out in
this study were FTIR and SEM. Based on the results of the study, the best membrane conditions were
obtained from composite membrane analysis with a tensile strength of 34,118 Mpa. In the Scanning
Electron Microscopy (SEM) analysis, the lowest composite membrane has a dense and homogeneous pore,
while the highest composite membrane has a hollow pore and has been homogeneous.
1 INTRODUCTION
The composite membrane consists of organic
polymers and inorganic fillers which can improve
the performance of membranes eg, zeolites have
been added into polymer membrane to increase
selectivity of gas separation. Silica is one of the
abundant metal oxides in volcanic ash can be
utilized as the basic material of silica gel synthesis
through the formation of alkali silicate precursors.
Sodium silicate can be converted into silica gel by
condensation process and hydrolysis using solvent,
both polar and non polar. By extracting the silica in
the alkaline state so that sodium silicate will form.
Sodium silicate will undergo polymerization process
to form silica gel on some difference of pH and
solvent (Uhlmann and Kreidhl, 1980). Silica is use
to reduce excessive swilling as to control the
moisture content, reduce the permeability of
methanol, increase mechanical stability and the
conductivity of proton in PEM for fuel cell (Siniwi,
2014). The chitosan is attractive for use as a
membrane base material because it has a functional
group -NH
2
and -OH which is easy to modify.
Chitosan membranes are hydrophilic, non-toxic,
biodegradable, large surface area and reactive to
metal ions because they have an active group -NH
2
and -OH. Chitosan membranes also have
disadvantages of low mechanical properties
indicated by the price of tensile strength (tensile
strength), percent elongation (percent extension),
and low modulus young. The basic ingredients of
inorganic compounds are silica which aims to
improve the stability of the chitosan membrane
through the formation of crosslinks with silica
through the formation of hydrogen bonds between
the chitosan structure and the silica (Widhi
Mahatmanti, 2014). The selection of chitosan as an
alternative to modify the plastic cause chitosan has
biodegradable characteristic (Maulida, et,al., 2018).
(Neburchilov et al., 2007) reported that the addition
of nanosilika to the membrane nafion (nafion / SiO
2
)
for DMFC can increase proton conductivity by a
ratio of 0.33 to 0.38 compared to a membrane
nation. Volcanic ash or volcanic sand is a falling
volcanic material that is ejected into the air during
an eruption. Low-energy basal eruptions (basal: dark
Maulida, ., Sukeksi, L., Harahap, M., Ginting, M., Wici, H. and Ayu, G.
Effect of Addition Silica Gel from Volcanic Ash of Sinabung Mountains to Tensile Strength on Chitosan Composite Membrane.
DOI: 10.5220/0008886500570061
In Proceedings of the 7th International Conference on Multidisciplinary Research (ICMR 2018) - , pages 57-61
ISBN: 978-989-758-437-4
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
57
frosted rocks, fine-grained clays of lava from
volcanoes) produce a distinctively dark ash
containing 45-55% silica which is generally rich in
iron (Fe) and magnesium (Mg). Based on research of
(Nakada and Yoshimoto, 2014) stated that the silica
content in volcanic ash of Sinabung Mount was
58.10%. The high content of silica in volcanic ash of
Mount Sinabung is an interesting subject for further
investigation, especially regarding the use of
volcanic ash as the base material of silica adsorbent
to bind lead weight metal.
2 MATERIALS DAN METHODS
2.1 Raw Materials and Equipments
The material used chitosan was obtained from
Faculty of Mathematics and Natural Sciences, silica
was obtained from volcanic ash of Sinabung
Mountain, NaOH, aquadest, acetic acid
(CH
3
COOH) and hydrochloric acid (HCl) were
obtained from CV. Rudang Jaya The equipment
used in this research were hot plate, 50 mesh sieve
and 230 mesh sieve, filter paper, magnetic stirrer,
funnel, measuring cup, oven and glass beaker.
2.2 Preparation of Volcanic Ash
The volcanic ash was sieved with a 230 mesh sieve
to homogenize the ash size. The sifted ash was taken
as much as 50 grams and soaked with HCl and
filtered. Then the ash washed and dried with oven.
2.3 Extraction of Sodium Silica
Solution
Volcanic ash was dissolved with 500 ml of 4 M
NaOH and heated at 190 °C with a variation of 120
minutes. It was filtered to get the filtrate. The filtrate
was tested with gravimetric (Maulida, et.al., 2017).
2.4 Process of making Silica Gel
The sodium silica solution inserted into the beaker
glass. Then dripped with HCl with a variety of
concentrations of 8 M to form a white gel with pH of
7. Silica gel was precipitated 24 hours and filtered
with paper washed with aquadest. Silica gel was
dried using an oven at 100 °C to remove excess acid
(Maulida, et.al., 2017).
2.5 Process of Making Chitosan
Composite Membranes with Silica
Fillers
Composite membrane synthesis was carried out
using phase inversion method. First, 2 grams of
chitosan dissolved in 100 mL of 2% acetic acid at
room temperature for 2 hours. Second, silica
composition is 0.6, 0.9, 1.2 grams, respectively. then
stirred at a temperature of 600 °C for 8.12.16 hours.
The homogeneous solution is also referred to as a
dope solution which has no air bubbles, then the
filtered solution is then poured onto a 20 x 20 cm
glass meld and dried at room temperature resulting
in a dry membrane. Dry membrane soaked with 1 M
NaOH for 2 hours then washed with aquadest to
neutral pH and dried at room temperature.
3 RESULTS AND DISCUSSION
3.1 Characteristic of Fourier
Transform Infra - Red (FTIR)
Silica Gel
Characteristic of FTIR silica gel were carried out to
identify functional groups of silica gel. The
characteristics of FTIR silica gel can be see in the
figure 1.
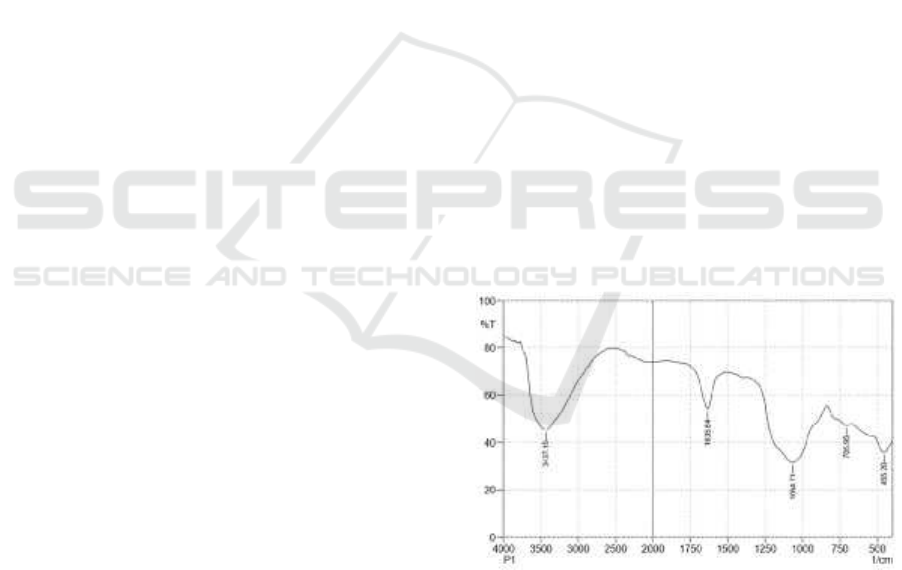
Figure 1: Characteristic of FTIR Silica Gel from Volcanic
Ash of Sinabung Mountain (Maulida,et.al.,2017).
The FTIR test on silica gel obtained by the
absorption peak at wave number 3437.15 cm
-1
shown the presence of silanol function group
derived from hydroxyl group bond Si-Si, at wave
number 1635.64 cm
-1
was indicated the presence of
hydroxyl (OH) group, on the wave number 1064.71
cm
-1
was indicated the presence of siloxane
ICMR 2018 - International Conference on Multidisciplinary Research
58
functional groups (Si-O-Si), at the wave numbers
705.95 cm
-1
and 455. 20 cm
-1
was indicated the
presence of Si-O functional groups (Silverstein,
1981).
3.2 Characteristic of Scanning Electron
Microscopy (SEM) Silica Gel from
Volcanic Ash of Sinabung
Mountain
Analysis of SEM was conducted to determine
membrane pores.
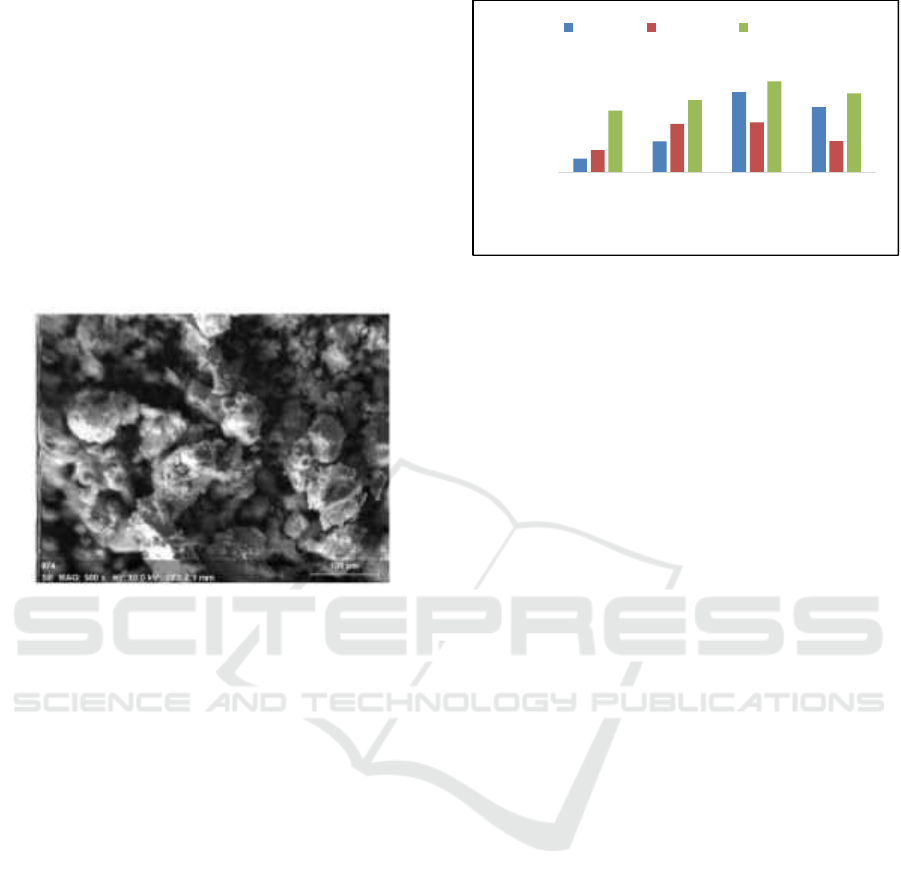
Figure 2 : Characteristic of Scanning Electron Microscopy
(SEM) Silica Gel from Volcanic Ash of Sinabung
Mountain.
In this study, it was cleared at the sample surfaces
that there were uneven and composed of clumps,
which has shown a wide variety of grains with
uneven distribution on the surface. The separation
between lumps is also seen quite clearly, it was
micro-cracking found among clusters (Maulida,
et.al.,2017).
3.3 The Effect of Silica Gel Mass
Variation and Stirring Time to
Tensile Strength of Composite
Membrane
Characteristics of mechanical properties need to
know the strength of membrane against forces
outside, which can damage the membrane. The
following graph shown the effect of silica variation
and stirring time to tensile strength of the composite.
Figure 3 : The Effect of Silica Gel Mass Variation and
Stirring Time to Tensile Strength Of Composite
Membrane.
Based on figure 3, the mass of silica and the length
of stirring time increase; the value of tensile strength
increases too. The results of this study was indicated
that at 8 hours of stirring time, the tensile strength of
membrane was 7,793 MPa on silica variation of 0.6
gram, then increased to 11,735 MPa at variation of
1.2 gram silica. At 12 hours of stirring time, tensile
strength was 18.260 MPa at 0.6 gram, then increased
to 23,140 MPa at variation of 1.2 grams of silica. In
the 16 hour stirring time, the membrane flux value
of 27.168 MPa at 0.6 grams, then increased to
34.118 MPa in a variation of 1.2 grams of silica.
From Figure 4, we can see the effect of adding silica
filler to the tensile strength of composite membrane.
The increased amount of silica can cause the tensile
strength of the membrane increased too. Tensile
strength is the maximum resistance that can be
retained by the material when given a force before
the material is broken. The more silica added to the
membrane, the more interaction between the silica
and the chitosan. This will lead to stronger bonds
and intermolecular forces in the membrane. The
addition of silica will cause crosslinks between silica
and chitosan by hydrogen bond (Karlina, 2016). The
tensile strength of this membrane has increased as
the percentage of silica increased. The more silica
added to the membrane, the more interaction
between the silica and the chitosan. This will lead to
stronger bonds and intermolecular forces in the
membrane. The addition of silica will lead to
crosslinking between silica and chitosan by
hydrogen bonding. The increase in tensile strength
in the addition of 0.6 grams of silica to 1.2 grams of
silica followed by a drop in tensile strength at the
next point of 1.5 grams of silica. The occurrence of a
drop in the value of tensile strength is likely due to
the excess amount of silica that causes during the
0
10
20
30
40
0.6 0.9 1.2 1.5
Tensile Strength (MPa)
Massa of Silica (gram)
8 hours 12 hours 16 hours
Effect of Addition Silica Gel from Volcanic Ash of Sinabung Mountains to Tensile Strength on Chitosan Composite Membrane
59

drying process of the cracked composite membrane.
This indicates that the increase in concentration does
not guarantee an increase in the value of tensile
strength.
Generally, the results show the greater filler to the
membrane and stirring time. They give higher
tensile strength results. Previous research on the
manufacture of composite membranes from chitosan
showed similar results where the tensile strength
value obtained was greater by increasing the
addition of silica, it is. due to its dense structure
which causes the distance between the molecules in
the membrane to be denser so as to have a large
tensile strength (Thermo, 2001).
3.4 Characteristic of Scanning Electron
Microscopy (SEM) Chitosan
Composite Membrane with Silica
Gel
An analysis of SEM was performed to determine
membrane pores. The test was performed at 0.6
gram silica fused chitosan membrane with 8 hours of
stirring time and composite membrane with silica
addition of 1.2 grams of silica with a stirring time of
16 hours. Because the composite membrane with 0.6
gram of silica filler has a low flux value, while 1.2
grams of silica has a high flux value.
Figure 4: Characteristic of Scanning Electron Microscopy
(SEM) Chitosan Composite Membrane with Silica Gel.
Figure 4 shows the result analysis SEM of the
membrane product with the addition of 1.2 grams of
silica and 16 hours of stirring time has a relatively
more dense morphology, which indicated that silica
was evenly mixed in the resulting membrane. The
addition of silica causes a dense chitosan membrane
to be hollow because the negative charge of the
chitosan OH-reacts with the silica so that it will
attract and form a small cavity (Siniwi, 2014). Based
on Figure 4 it can be seen that the pore size of the
membrane composites with 1.2 g silica added with
16 hours of stirring time was homogeneous. It’s due
to the addition of silica which made the membrane
structure hollowed with longer stirring time, so the
membrane becomes more homogeneous and can be
applied for filtration.
4 CONCLUSIONS
The addition of silica too much and string of time
too long causes the composite membrane to overlap
until homogeneous so that the tensile strength
increases, acetic acid is a good solvent use in
dissolving chitosan and silica, addition of the filler
on making composite membrane cause the greater
pore in membrane.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge that the present
research is supported by Ministry of Research and
Technology and Higher Education Republic of
Indonesia. The support is under the research grant
BP-PTN USU of Year 2016 Contract Number
XXX/XXX.
REFERENCES
D.R. Uhlmann, and N. J. Kreidhl., 1980. “Glass Sciences
and Technology”. Academy Press.
Karlina, Yhuni. 2016. Peran Abu Vulkanik sebagai Filler
Pada Sintesis Polymer Electrolyte Membrane (PEM)
Terbuat dari Chitosan. Fakultas Matematika dan Sains.
Universitas Negeri Semarang.
Maulida, G. Melva., W, Herlinawati., 2017. Extraction
Volcanic Ash of Sinabung Mount Silica to Production
Silica Gel. J. Teknik Kimia USU.
Maulida, Harahap, Mara Bangun., Alfarodo, Anita
Manulang and Ginting, M.H.S., 2018. Utilization of
Jackfruit Seeds (Artocarpus Heterophyllus) in The
Preparing of Bioplastics by Plasticizer Ethylene Glycol
and Chitosan Filler. Journal of Engineering and
Applied Sciences. Asian Research Publishing Network.
Nakada,S and M. Yoshimoto., 2014. Earthquake Research
Institute, University of Tokyo http://www.eri.u-
tokyo.ac.jp/en/2014/02/04/eruptive-activity-of-
sinabung-volcano-in-2013-and-2014.
Neburchilov, V., J. Martin, H. Wang and J. Zhang., 2007.
Polymer Electrolyte Membranes for Direct Methanol
Fuel Cell, Journal of Power Sources.
Widhi Mahatmanti, F., 2014. Physical Characteristics of
Chitosan Based Film Modified With Silica and
ICMR 2018 - International Conference on Multidisciplinary Research
60

Polyethylene Glycol. Indo. J. Chem. ResearchGate.
Silverstein, R.M., Basslerr, G.C., and Morrill, T.C., 1981.
Spectrometric Identification of Organic Compounds,
John Wiley and Sons. Ney York, 4
th
edition.
Siniwi,,Widasari Trisna., 2014. Sintesis dan Karakterisasi
Proton Exchange membrane Kitosan- Nanosilika.
Jurusan Kimia, Fakultas Matematika dan Ilmu
Pengetahuan Alam, Universitas Negri Semarang.
Thermo Nicolet., 2001. Introduction to Fourier Transform
Infrared Spectrometry. Thermo Nicolet Corporation :
Madison – US.
Effect of Addition Silica Gel from Volcanic Ash of Sinabung Mountains to Tensile Strength on Chitosan Composite Membrane
61
