
Effect of Various Feeding of Live Feeds on the Growth and Survival
Rate of Black Tiger Shrimp Larvae (Penaeus Monodon)
Dedi Fazriansyah Putra
1
, Mariana
1
, Muchlisin Zainal Abidin
1
and Sanani
2
1
Department of Aquaculture, Syiah Kuala University, Banda Aceh, Indonesia
2
Center for Brackishwater Aquaculture, Ujung Batee, Aceh Besar District, Indonesia
Keywords: Live Feeds, Black Tiger Shrimp (Penaeus Monodon), Growth, Survival Rates.
Abstract: The aim of this research was to investigate the effect of variousfeeding of live feeds on the growth and
survival rates of black tiger shrimp larvae (Penaeus monodon). The complete randomized experimental
design was used with 4 treatment levels and 4 replications. The live feed treatments were (A) Chlorella sp.,
(B) Tertraselmis chuii, (C) Spirulina sp and (D) Skeletonema costatum. The larvae were fed with 120,000
cells/ml with feeding frequency of 4 times daily for 11 days. The results showed that different live feed had
significant effect on weight gain, length gain, daily growth rate, specific growth rate and survival rate.
Duncan's further test showed that the optimum weight gain, length gain, daily growth rate, specific growth
rate and survival rate were found in treatment D (Skeletonema costatum) with 0,0006±0,0008gram,
3,94±0,15 mm, 0,0005±0,0001g/day, 14,98±2,47 %/day and 83,50±5,29 %, respectively. Thus, it is
concluded that the recommended live feed for black tiger shrimp larvae feeding was Skeletonema costatum.
1 INTRODUCTION
As a tropical country, Indonesia has abundance of
fisheries resources and natural diversity (Muchlisin
et al., 2016; Muchlisin et al., 2017). Black tiger
shrimp (Penaeus monodon) is one of the leading
non-oil export commodities of fishery aquaculture
sector (Raya, 2011). The cultivation of black tiger
shrimp has become one of the industries in tropical
and subtropical countries (Adger, 1998). As one of
tropical country, Indonesia has reached its heyday in
maintaining black tiger shrimp. Unfortunately, the
production of black tiger shrimp in Indonesia is
declining nationally, as illustrated in 1995 the
production reached 180,000 tons but fell to 125,000
tons in 2000 (Soetrisno, 2004) Penaus monodon is
an active organism foraging at night (noctural), the
type of feed varies greatly depending on the level of
its life cycle, and the larval stadia. The main food is
the type of plankton (phytoplankton and
zooplankton), but in the adult stadium the tiger
prawn likes softfood or mollusk (clams, oysters,
snails), polychaeta worms, and crustaceans
(Soetrisno, 2004). In intensive and semi-intensive
cultivation ponds, in addition to commercial feeds,
the tiger shrimps also obtain live feeds growing in
ponds, among others, moss, plankton, and benthos.
But if the lack of food occurs, they will be cannibal
in other small or weak shrimps especially during the
turn of the skin or moulting (Rothlisberg, 2000).Live
feeds such as phytoplankton and zooplankton are
commonly given in shrimp larval culture to post-
larval stadia (FAO, 2013). Penaeid larvae are mostly
cultured on live unicellular algae during the
protozoea stadia. Algaes are shortly consumed from
zoea 1 until about postlarvae (PL) 2. The target
density for algae such as Chaetocerosmuelleri or
Skeletonemacostatum is 100 000 cells/ml as the only
algae fed.
We used several potential live feeds for black
tiger shrimp larval in this experiment such
asChlorella sp.Tetraselmischuii,Spirulina sp.
andSkeletonemacostatum. Chorella sp. is a single-
celled green algae and the cell wall is thin, rather
hard, solid, and 3-8 microns long. Chlorella sp.is
very suitable to be consumed by the fish larvae,
apart from that Chlorella sp. containing 30% protein
and 15% lipid, but it also contains carotene pigment
in the form of lutein (Bachtiar, 2003). Tetraselmis
chuii is a single-celled organism including plant
species, whose body size is 7-12 microns. Chitri
chitiel has high glycemic content, 54.66% protein,
18.31% carbohydrate and 14.27% fat. Chlorophyll
chlorophyll pigments consist of two kinds, namely
128
Putra, D., Mariana, ., Abidin, M. and Sanani, .
Effect of Various Feeding of Live Feeds on the Growth and Survival Rate of Black Tiger Shrimp Larvae (Penaeus Monodon).
DOI: 10.5220/0008883001280132
In Proceedings of the 7th International Conference on Multidisciplinary Research (ICMR 2018) - , pages 128-132
ISBN: 978-989-758-437-4
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

carotene and xanthophylls (Bachtiar, 2003).
Spirulina sp. is a green algae that is classified
into Cyanobacteria, one-celled and spiral-shaped.
Based on its habitat, spirulina sp.it can thrive in
tropical and subtropical waters. (Chen Y.Y. et.
Al,2016) explains that spirulina contains five main
nutrients: carbohydrate, protein, fat (Gama Linoleat,
Omega 3, 6, and 9), vitamins (B-complex, E),
minerals (Fe, Ca, k), as well as natural pigments
(Beta carotene, chlorophyll, Xantofil, Fikocyanin).
The cell is 1-3 microns in diameter, and Spirulina
sp.containshigh protein and lipid (Chen Y.Y.et. al.
2016). Skeletonema costatum
is a phytoplankton of a
single-celled diatomae type and cell size ranges from
4-15 μm, Skeletonema costatum is widely used in
shrimp culture due to its high nutrient content, which
is 30.35% protein, 1.55% fat. Based on above
description,in this study, we would like to
investigate the effect of different live feeds feeding
on the growth performance and survival rate of
black tiger shrimp.
2 METHODOLOGY
2.1 Experimental Procedure
Black tiger shrimp, Penausmonodon larvae were
obtained from Center for Brackishwater Aquaculture
Ujung Batee, Aceh Besar District, Indonesia. The
experimental system consisted of 16 aerated tanks
(25 l volume) of 100 shrimp larvae used within each
tank (10 l). Shrimp’s larvae used were ranging from
Stadia zoea to Stadia mysis. Shrimps were fed four
times daily at 08.00; 12.00, 16.00, 20.00 for eleven
days. Penausmonodon larvae were fed 120,000
cells/ml (Panjaitan et al., 2014) of following
treatments:
Treatment A: Chlorella sp.
Treatment B: Tetraselmis chuii
Treatment C: Spirulina sp.
Treatment D: Skeletonema costatum.
2.2 Observation Parameters
The following variables were calculated:Survival
Rate (SR)was calculated using the formula (Putra et.
Al, 2016).
SR =
x100
(1)
Information :
SR = Survival Rate (%) Nt = Final number of live
shrimp
No = Initial number of live shrimp
Weight gain (WG), calculation of absolute weight
growth using the formula Steffens (1989) as follows:
∆G= W
t
-Wo (2)
Information:
ΔG = weight gain (g) Wt = Weight of shrimp at end
of experiment (g) Wo = Shrimp weight at the
beginning of experiment (g)
Length gain (LG) of shrimp was calculated by the
formula (Putra et al., 2016)
L= L
t
-Lo (3)
Information:
ΔL = increase Absolute length (cm) Lt = Average
length of research (cm)
Lo = average length of initial study (cm)
Specific growth rate (SGR)the calculation of
specific growth rates (Steffens, 1989) as follows:
0
100
(4)
Information:
SGR = Specific growth rate (% / day) Wt = Shrimp
biomass test at end of study (g) W0 = Shrimp
biomass test at start of study (g) t = Maintenance
time (day)
Daily growth rate (DGR), the daily growth rate
according to Steffens (1989) as follows:
DGR =
(5)
information:
DGR = Daily growth rate (g / day) Wt = The weight
of shrimp biomass test at the end of the study (g);
W0 = The weight of shrimp biomass test at baseline.
2.3 Statistical Analysis
One way ANOVA and Duncan’s multiple range test
(Duncan. 1955) was used to investigate the
significance of the difference among the means of
treatments through SPSS version 22.
3 RESULT
The results showed that the increase of tiger shrimp
weight gain (WG) ranged from 0.0002±0.0002gram
to 0,0006±0.0008 gram, length gain(LG) ranged
from 1,54±0,02mm to 3,94±0,15mm,daily growth
rate (DGR) ranged from 0,0002±0,0001 gram/day to
0,0005±0,0001 gram/day the specific growth rate
(SGR) ranged from 8,95±5,20%/day to 14,98±2,47
Effect of Various Feeding of Live Feeds on the Growth and Survival Rate of Black Tiger Shrimp Larvae (Penaeus Monodon)
129
%/day, and the survival rate (SR) ranged from
35,55±3,61 % to 83,50±5,29 %(Table1& 2).
The results of ANOVA (Analysis of Variant)
showed that live feed had significant effect on
weight gain, length gain,daily growth rate, specific
growth rate, and survival rate of black tiger shrimp
larvae (P <0,05). Duncan's advanced test showed
black tiger shrimp fed with Skeletonemacostatum
(treatment D) showed the best performance of
weight gain, length gain, daily growth rate, specific
growth rate and survival rate.
Table 1: Weight gain (WG), Length gain (LG) of black
tiger shrimp larvae (P. monodon) for 11 days feeding.
Treatment WG (g) LG (mm)
Chlorella sp.(A) 0,0003±0,0001
b
2,29±0,03
b
Tetraselmischuii (B) 0,0005±0,0001
c
3,54±0,073
c
Spirulinasp.(C) 0,0002±0,0002
a
1,54±0,02
a
Skeletonemacostatum (D) 0,0006±0,0008
d
3,94±0,15
d
Table 2: Daily growth rate (DGR), Specific growth rate
(SGR) and Survival rate (SR) of black tiger shrimp larvae
(P. monodon) for 11 days feeding.
Treatment DGR (g/day) SGR (%/day) SR (%)
Chlorella sp.(A) 0,0002±0,0001
b
10,91±2,72
b
35,55±3,61
b
Tetraselmischuii (B) 0,0004±0,0002
c
13,88±4,11
c
66,72±5,54
c
Spirulinasp (C) 0,0002±0,0001
b
8,95±5,20
a
55,52±7,08
a
Skeletonemacostatum (D) 0,0005±0,0001
d
14,98±2,47
d
83,50±5,29
d
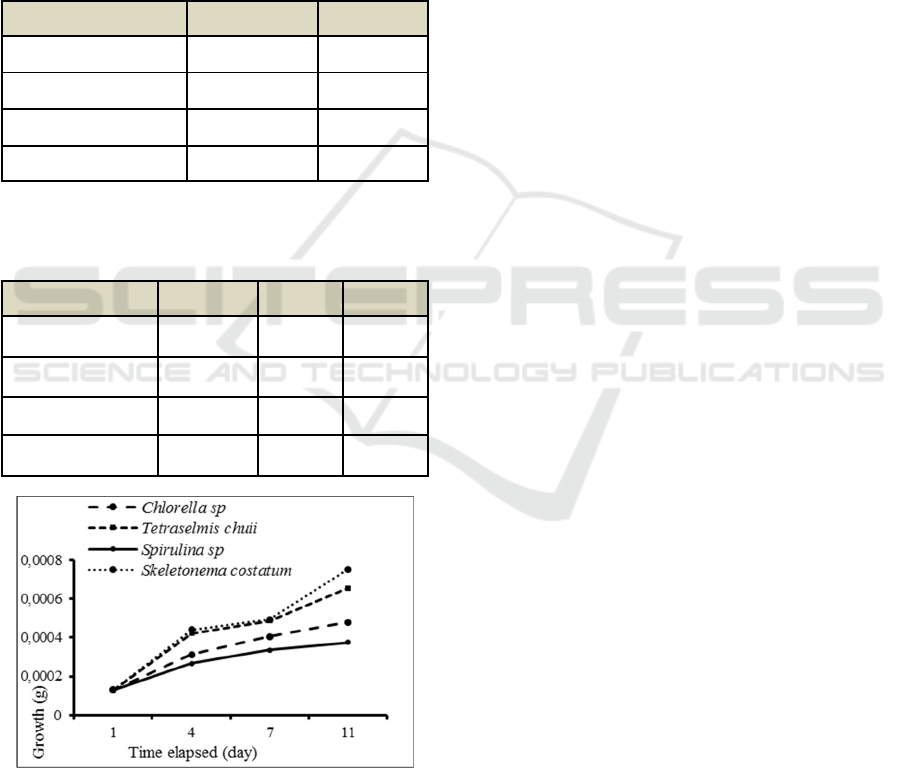
Figure 1: the growth of black tiger shrimp for 11 days
feeding.
The highest growth performance was found at
shrimp fed with skeletonemacostatum as seen at
Figure 1. The result also indicated that better
weightgain was found in treatment D with
skeletonema costatum feed type.
Water quality measurements showed a pH
ranging from 7.9 to 8.8, temperatures ranging from
27
o
C-30.05
o
C, DO ranging from 5.00ppm-6.07 ppm
and salinity ranging from 25-35 ppt (data not
shown).
4 DISCUSSION
The results showed that feeding of different live
feeds expressed significant effect on weight gain,
length gain, daily growth rate, specific growth rate,
and survival rate on black tiger shrimp larval. This is
in accordance with Rothlisberg (1998) stating that
shrimp protozoa stages feed mostly on
phytoplankton and small zooplankton where it
developed from mysis through to postlarva (PL), and
there was a transition to active predation on larger
zooplankton (Lovett & Felder, 1990).
In aquaculture activities, particularly in shrimp
culture, the use of live feed at larval stadia to
stimulate the growth performance is highly
recommended (Fitriani et al., 2017). We found that
feeding Skeletonema costatum showed the best
growth performances on Penausmonodon. We
assumed that Skeletonema costatummeet the
nutritional needs of tiger shrimp larvae. Studies
about shrimp nutrition especially on shrimp lipids
have shown that shrimp absolutely require essential
fatty acids (EFA) for their normal growth
development (Liao & Liu, 1989). S. costatum is a
phytoplankton from diatome that is suitable given in
mysis stadia, single cell and small cell size ranging
from 4-15 μm, containing 32.05% protein, 7% fat,
2.09% fiber, 44.37 ash % and water content of
8.41% (Erlina et al., 2004). In addition to ,S.
costatum has a thin cell wall that is easily digested
by shrimp larval. It has autolysis enzymes that can
help digestion, small cell size in accordance with
opening the mouth of the larvae, moving actively
that can attract shrimp larva to catch the algae
(Bachtiar. 2003). In terms of color,S. costatum has a
brownish color that attracts shrimp attention to eat it.
S. costatum is widely used in other penaid
culture (Stafford. 1999). Beside as feed suplement,
S. Costatumalso avalaible
in
dried form (Lestari et
al., 2014). The application of microalgae and
macroalgae in shrimp culture does not only stimulate
the growth performance but also shrimp immune
system. Several studies have been conducted
showing that the dietary administration of
microalgae and macroalgae in shrimp diet can
ICMR 2018 - International Conference on Multidisciplinary Research
130

significantlyaffect the immune system (Chen et al.,
2016; Chen et. al. 2014; Kitikiew et al., 2013; Lin et
al., 2013).
The shrimp mortality during the study was varied
from 35-83 %. We assumed that it was due to the
moulting activity when growing up. When moulting
occurs, shrimp body resistance weakens and appetite
decreases so that larvae will be more passive and
may cause cannibalism by healthy shrimp.
Water quality parameters are important factors
that must be considered in the maintenance of tiger
shrimp larvae. Water quality is closely related to the
growth and survival of tiger shrimp larvae. In this
study, the water quality has not changed
significantly, therefore the water quality in the
rearing area is still in the normal range. The
measured water temperature ranges from 27
o
C-30
o
C,
the measured water pH ranges from 7.9-8.7, DO
ranges from 5.00ppm-6.07ppm and the measured
salinity ranges from 25-34ppt. According to Boyd
(1989) the optimal temperature of shrimp larvae
growth between 26-32 0C, optimal pH range of tiger
shrimp larvae maintenance between 7,8-8,8 and
optimal salinity range of shrimp larvae 24 ppt-35
ppt.
5 CONCLUSION
Based on the result, it is concluded that different live
feed feeding has significant effect on growth
performance and survival rate of black tiger shrimp
(P.monodon), where the recommendedlivefeed in
this research is Skeletonema costatum.
REFERENCES
Adger W. 1998.Sustainability and social resilience in
coastal resources use. In: GEC-1997-23, CSERGE
working paper
Bachtiar, Y. 2003. Menghasilkan pakan alami untuk ikan
hias. Agromedia Pustaka. Jakarta, 10-14.
Boyd, C.E. 1989. Water quality management and aeration
in shrimp farming.Fisheries and Allied Aquacultures
Department Series No. 2, Alabama Agricultural
Experiment Station, Auburn University, Alabama
Birmingham. Publishing Co., 183 pp.
Chen, Y.Y. et. al. 2014.Shrimp that have received
carrageenan via immersion and diet exhibit
immunocompetence in phagocytosis despite a post-
plateau in immune parameters, Fish. Shellfish
Immunol. 36:352-366
Chen, Y.Y. et al. 2016.Spirulina elicits the activation of
innate immunity and increases resistance
against Vibrio alginolyticus in shrimp. Fish Shellfish
Immunol., 55: 690-698.
Duncan, D. B. 1955. Multiple Range and Multiple F Tests.
Biometrics 11:1
D'Abramo, L.R. 1989. Lipid requirements of shrimp. In
Advances in Tropical Aquaculture, Tahiti, February 20
– March 4, 1989, pp. 277–285. AOUACOP
IFREMER, Actes de Colloque 9.
D’Souza, F.M.L., et al. 2002. Flocculated microalgae
concentrates as diets for larvae of the tiger praw
Penaeusmonodon Fabricius. Aquaculture Nutrition, 8:
113–120.
Erlina, A. et. al. 2004.Kajian nutritive
fitoplanktonpakanalamipada system kultasimassal,
BalaiBesarPengembanganBudidaya Air Payau, 9(4):
206-210.
Evans, L.W. 1992. The establishment of a
commercial Penaeusmonodon prawn farm in
Zululand, South Africa. In T. Hecht & P. Britz, eds.
Aquaculture ’92. Proceedings of the Aquaculture
Association of Southern Africa, 1: 109–116.
Fitriani, et al. 2017.Pengaruhpemberianpupukanorganik
(NPK+Silikat)
dengandosisberbedaterhadapkepadatanSkeletonemac
ostatumpadapembenihanudangwindu.Akuatikisle:
JurnalAkuakultur, PesisirdanPulau-Pulau Kecil, 1(1):
11-18. https://dx.doi.org/10.29239/j.akuatikisle.1.1.11-
18
FAO 2013. On-farm feeding and feed management in
aquaculture, by Hasan, M.R.; New, M.B. Fisheries
and Aquaculture Technical Paper.No. 583.67 pp.
FAO. 1985a. Shrimp hatchery design, operation and
management, by P. Kungvankij, L.B. Tiro, Jr., B.J.
Pudadera, Jr., I.O. Potestas, K.G. Corre, E.
Borlongan, G.A.Talean, L.F. Bustilo, E.T. Tech, A.
Unggui& T.E. Chua. NACA Training Manual Series
No. 1, 95 pp. Bangkok, Network of Aquaculture
Centres in Asia, Regional Lead Centre in the
Philippines (available at http://www.fao.org/docrep/
field/003/ac232e/AC232E00.htm).
FAO. 1985b. A Prototype warm water shrimp hatchery,
by P. Kungvankij, L.B. Tiro, B.J. Pudadera, E.
Borlongan, E.T. Tech & T.E. Chua, Technology
Series No. 2, 19 pp. Tigbauan, Philippines
Aquaculture Department, Southeast Asian Fisheries
Development Center (available
at http://www.fao.org/docrep/field/003/ac234e/ac234e
00.htm).
Glencross, B.D., et al. 2002. The effect of dietary n–3 and
n–6 fatty acid balance on the growth of the prawn
Penaeusmonodon. Aquaculture Nutrition, 8: 43–52.
Guary J.C., et. al. 1976.The effects of a fat–free diet and
compounded diets supplemented with various oils on
moult, growth and fatty acid composition of prawn,
Penaeusjaponicus Bate. Aquaculture, 7: 245–254.
Kanazawa, A., et. al. 1977. Nutritional requirements of
prawn – VII.Effect of dietary lipids on growth.
Bulletin of the Japanese Society of Scientific
Fisheries, 43: 894–856.
Effect of Various Feeding of Live Feeds on the Growth and Survival Rate of Black Tiger Shrimp Larvae (Penaeus Monodon)
131

Kanazawa, A. 1984.Nutrition of penaeid prawns and
shrimps. In SEAFDEC Aquaculture Department,
ed. Proceedings of the First International Conference
on the Culture of Penaeid Prawns/Shrimps, pp. 124–
130. Iloilo City, Philippines.
Kitikiew, S. et. al. 2013.Fucoidan effectively provokes the
innate immunity of white shrimp Litopenaeusvannamei
and its resistance against experimental Vibrio
alginolyticus infection, Fish. Shellfish Immunol. 34:
280-290.
Kumlu, M. 1997a. Larval growth and survival of
Penaeusindicus (Decapoda: Penaeidae) on live feeds.
Turkish Journal of Biology, 22: 235–245.
Kumlu, M. 1997b. Feeding and digestion in larval
decapod crustaceans. Turkish Journal of Biology, 23:
215–229.
Kumlu, M. 1998. Larval growth and survival
of Penaeusindicus (Decapoda: Penaeidae) on live
feeds. Turkish Journal of Biology, 22: 235–245.
Lestari, D.P., et. al. 2014.Dried Skeletonemacostatum in
Feed Formulation for the Growth of Vaname Shrimp
(Litopenaeusvannamei). J. Exp. Life Sci. 4(2): 45-49
Liao, I.C. & Liu, F.G., 1989. A brief review of nutritional
studies for Penaeusmonodon. In Advances in tropical
aquaculture, Tahiti, February 20 – March 4, 1989, pp.
355–380. AQUACOP IFREMER, Actes de Colloque,
9
Lin,Y.C. et al. 2013.Vaccination enhances early immune
responses in white shrimp Litopenaeusvannamei after
secondary exposure to Vibrio alginolyticus, PLoS
ONE 8: e69722.
Lovett, D.L. & Felder, D.L. 1990.Ontogeny of kinematics
in the gut of the white shrimp Penaeussetiferus
(Decapoda; Penaeidae). Journal of Crustacean
Biology, 10: 53–68.
Merican, Z. O. & Shim, K.F. 1996.Qualitative
requirements of essential fatty acids for juvenile
Penaeusmonodon. Aquaculture, 147(3/4): 275–291.
Muchlisin Z. A., et. al. 2016.Inshore migration of tropical
glass eels (Anguilla spp.) in Lambeso River, Aceh
Jaya District, Aceh Province, Indonesia. Aceh Journal
of Animal Science 1(2):58-61.
Muchlisin Z. A., et. al. 2017.Fish fauna of Lake
LauikTawar and Lake Laulo, Simeulue Island,
Indonesia.Biodiversitas 18(2):752-757.
Panjaitan, S.A., et. al. 2014.Pemeliharaan larva udang
vannamei (litopenaeus vannamei, boone 1931) dengan
pemberian jenis Fitoplankton yang berbeda, Jurnal
Manajemen Perikanan dan Kelautan, 1(01):33-43.
Pina, P., et. al. 2006.Survival, development and growth of
the Pacific white shrimp Litopenaeusvannamei
protozoea larvae, fed with monoalgal and mixed diets.
Aquaculture, 253(1): 523–530.
Putra D. F., et al. 2016.Growth performance and survival
rate of climbing perch (Anabas testudineus) fed
Daphnia sp. enriched with manure, coconut dregs
flour and soybean meal. AACL Bioflux 9(5):944-948.
Raya, R. 2011. Model Pertumbuhan udang windu
(Penaeus monodon) untuk menentukan pemanen
optimum, Paradigma, 15(2):113-123.
Rothlisberg, P.C. 1998. Aspects of penaeid biology and
ecology of relevance to aquaculture: a review.
Aquaculture, 164(1): 49–65.
Soetrisno, C. K. 2004. Mensiasatipenyakit WSSV di
tambak. Aquaculture Indonesia, 5(1): 19-31.
Stafford, C., 1999. A Guide To Phytoplankton of
Aquaculture Ponds; Collection, Analysis and
Identification. Brisbane: The State of Queensland,
Department of Primary Industries, 1-65 p., ISBN: 0
7345 0029 7.
Steffens, W. 1989.Principles of Fish Nutrition. Chichester:
Ellis Honvood.
Xu, X.L., et. al. 1993.The nutritional value of dietary n–3
and n–6 fatty acid for the Chinese prawn
(Penaeuschinensis). Aquaculture, 118: 277–285.
ICMR 2018 - International Conference on Multidisciplinary Research
132
