
Butyrate Acid as a Potential Marker for Diversity of Gut Microbiota
in Colorectal Cancer Patients
Fauzi Yusuf
1
, Azzaki Abubakar
1
, Desi Maghfirah
2
and Siti Adewiah
2
1
Division of Gastroenterology-Hepatology, Department of Internal Medicine, Faculty of Medicine University of Syiah
Kuala/ Dr. Zainoel Abidin Centre Hospital, Banda Aceh, Indonesia.
2
Department of Internal Medicine, Faculty of Medicine, Syiah Kuala University, Banda Aceh, Indonesia.
Keywords: Colorectal Cancer, Butyrate Acids, Biomarker
Abstract: The gut microbiota acts as a real organ and many changes in its composition have been reported in
colorectal cancer (CRC). Short-chain fatty acids (SCFA) mainly produced as microbial metabolites, acetate,
propionate, and butyrate acids. Butyrate is produced by specific bacteria, mainly in the colon, and is taken
up by the host. In our study, we found that CRC patients had lower level of acetate, propionate and butyrate
acids than non-CRC. The mean concentration of acetate 8,55 µg/mL, propionate 5,61 µg/mL and butyrate
acids 3,79 µg/mL respectively. In three of SCFA, the level of butyrate acids had the best diagnostic
properties with area under receiver operating characteristic (ROC) curve of 0.84 higher than acetate (0.71)
and propionate (0.75) (p< 0.05).
1 INTRODUCTION
Colorectal cancer is the fourth most cancer evident
worldwide (Iffrig and Weinber, 2009). The rate of
colorectal cancer is 5-10 times higher in the most
developed country (Ginsberg et al, 2010). In
Indonesia, colorectal cancer disease is caused the
increasing evidences of cancer-related mortality in
recent years Based on epedimiological report during
1996-1999 from Pathology Anatomy Division of
Medical Faculty, Indonesia University, wrote the
colorectal cancer patients with age under 40 years
are around 36.75% ( Sudoyo et al, 2010). Colorectal
cancer is a disease from an accumulation of genetic
mutation, epigenetic, disregulate in their
communication in signaling pathways, and gut
microbial contribution. Microbial involvement in
colorectal cancer (CRC) is now well established
(Sekirov et al, 2010).
SCFA are the main products of anaerobic
microbial fermentation in the large intestine and
affect colonic health. SCFA mainly produced as
microbial metabolites, acetate, propionate, and
butyrate acids. The formation of butyrate and other
SCFA possibly playing a major role as
chemopreventive products of microbial fermentation
in the colon (Faujan et al, 2010; Topping and
Clifton, 2001). Butyrate production is a major source
of energy for colonic epithelial cells and affecting to
the protection from colitis and colorectal cancer (
Rose et al, 2007; Vinolo et al, 2011). The relation
between functional characteristics, such as SCFA
especially butyrate acid and CRC has not been
extensively investigated. In one study showed
butyric acid was significantly higher in the feces of
healthy subject than CRC ( Simpson and Campbell,
2015). In this study, we evaluated the level of
butyrate acid as diagnostic biomarkers for diversity
of gut microbiota in colorectal cancer patients.
2 PATIENTS AND METHODS
2.1 Participants and Sample Collection
The study consists of fourteen subjects with CRC
and 14 non-CRC were from in the Gastroenterology-
Hepatology Department at Dr. Zainoel Abidin
General Teaching Hospital Banda Aceh, Indonesia.
The patients were selected based on the following
inculsion criteria :1) Patients aged 18 years or over ;
2) Indonesian citizen that proved by identity cards;
3) Patients wtih colorectal cancer confirmed by
pathological examination; 4) Patients instead of
CRC; and 5) Patients who are able to cooperate in
Yusuf, F., Abubakar, A., Maghfirah, D. and Adewiyah, S.
Butyrate Acid as a Potential Marker for Diversity of Gut Microbiota in Colorectal Cancer Patients.
DOI: 10.5220/0008790200270030
In Proceedings of the 2nd Syiah Kuala International Conference on Medicine and Health Sciences (SKIC-MHS 2018), pages 27-30
ISBN: 978-989-758-438-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
27
the study. None of the patients had active antibiotic
treatment or within the month prior to the
colonoscopy, youghurt consumtion or laxative
medicine for the last five weeks and none were
treated with chemotherapy and/or radiotherapy in
the previous six months. One stool samples was
collected from each participant, where stool samples
will be labelled and stored at -20ºC freezer service
by the researchers. The study was approved by the
Ethical Review Committee of Medical Faculty,
Syiah Kuala University, Banda Aceh, Indonesia.
2.2 Gas Chromatography Analysis of
Fecal SCFA Concentration
Stool samples were analysed for SCFA
concentration with gas chromatography (GC) as
described from a previous methods (Chen et al,
2013; Tangerman and Nagengast, 1996]. The
amounts of acetate, propionate and butyrate acids
have been reported as µg/ml and %.
2.3 Statistical Analysis
The exact chi-square test and Student t-test were
used for the comparisons between the groups, and
nonparametric statistics was used in addition for
variables without normal distribution. The
diagnostic properties were assessed with Receiver
Operating Characteristics (ROC) curves, sensitivity,
specificity, positive- and negative Likelihood Ratio
(LR) and Diagnostic Odds Ratio (DOR). P-values
below 0.05 were judged as statistically significant
3 RESULTS
3.1 Participants
Total 28 participants were included in this study.
Table 1 shows the characteristics of participants.
There were 10 males and 4 females in the CRC
group, and 9 males and 5 female in non-CRC group.
The means (±SD) was 53.8± 13.3 years for CRC and
50.0 ± 17.6 years for non-CRC. Haemoglobin, BMI
and albumin in the CRC group was lower than non-
CRC group. Percentage of cancers based on
location: rectum 79% and colon descending 21%.
Table 1 Characteristic of participants in this study
Variable Colorectal
cancer
(% or SD)
Non – colorectal
cancer
(% or SD)
Gender
Male
Female
10 (72%)
4 (28%)
9 (64%)
5 (36%)
Age (years, mean)
53.8 ± 13.3 50.0 ± 17.6
BMI (kg/m
2
,
mean)
20.21 ± 2.65 23.6 ± 1.91
Hemoglobin (g/dl,
mean)
10.6 ± 2.1 12.3 ± 1.2
Albumin (g/dl,
mean)
3.24 ± 0.71 3.91 ±0.53
Colonoscopy
Ca Rectum
11 (79%)
Ca Colon
Descending
3 (21%)
Colitis Infection 10 (72%)
ColitisInfection
+ Hemorrhoid
Interna
4 (28%)
The mean faecal concentrations of acetate,
propionate dan butyrate were significanty lower in
patients with CRC compared non-CRC. From the
table 2, results revealed that the mean concentration
of acetate 8,55 µg/mL, propionate 5,61 µg/mL and
butyrate acids 3,79 µg/mL respectively (all P <
0.05).
Table 2. Fecal short-chain fatty acids in subjects with and
without colorectal cancer.
Variable
Colorectal
cancer
patients
(N=14)
Non-colorectal
cancer patients
(N=14)
P-
value
Acetate
Acids
8.55 ± 3.06 11.78 ± 4.61
0.038
Propionate
Acids
5.61 ± 1.95 8.61 ± 3.40
0.008
Butyrate
Acids
3.79 ± 2.04 6.81 ± 2.59
0.002
The results are given as mean values with SD.
3.2 Diagnostic Properties of SCFA
In three of SCFA, the level of butyrate acids had the
best diagnostic properties with area under receiver
operating characteristic (ROC) curve of 0.84 higher
than acetate (0.71) and propionate (0.75) (p< 0.05)
(Table 3, Figure 1).
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
28
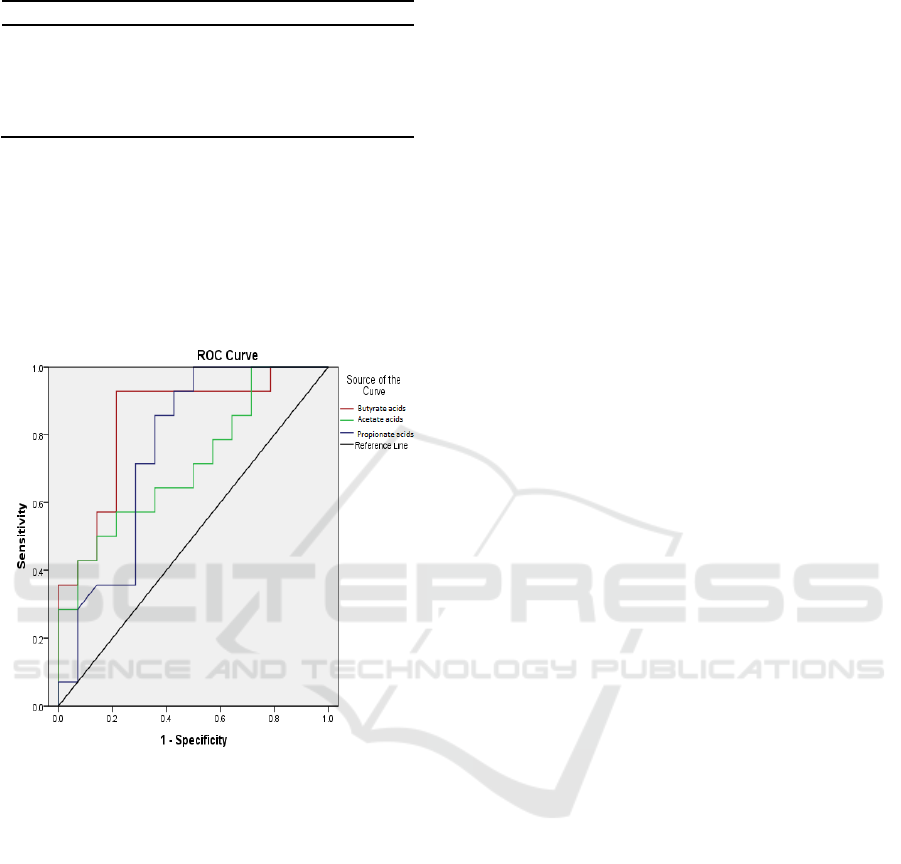
Table 3. Area under curve (AUC) short chain fatty acids.
AUC 95%CI
Acetate acids
Propionate acids
Butyrate acids
0.71
0.75
0.84
0.52 – 0.90
0.57 – 0.94
0.68 – 0.99
Butyrate acid value < 5.4 µg/mL was judged
as a well-suited cut-off for indicating CRC and was
used in the calculation of the diagnostic. According
to the reference range employed in this study, the
sensitivity, specificity, positive and negative
likelihood ratio, and diagnostic odds ratio were 85%,
78%, 4.04, 0.18 and 22.2 respectively.
Figure 1: The diagnostic properties of SCFA presented as
the Receiver Operating Characteristic curve.
4 DISCUSSION
With the increasing evidence linking gut microbiota
and CRC, fecal microbiota has emerged as a
promising candidate to non-invasively screen for
CRC. The present study demonstrated that the level
butyrate acids could be a valuable diagnostic
biomarker for CRC than the other SCFA.
Colorectal cancer (CRC) is a leading cause
of cancer-related mortality worldwide, and its
incidence has increased rapidly in recent years
(Jemal et al, 2011; Ji et al, 1998). In recent years,
the 16S rRNA gene sequencing approach has been
widely used as an effective tool to globally analyze
the microbial community, and multiple studies have
demonstrated that breakdown of the intestinal
microbiota structure can promote carcinogenesis and
development of CRC (Wei et al, 2016). Flanagan et
al, 2014 . demonstrated a significant association
between Fusobacterium nucleatum level and patient
outcome and suggested that F. nucleatum may have
value as a prognostic indicator. Boleij et al, 2015.
found that the detection of Bacteroides fragilis toxin
(BFT), which was produced by Enterotoxigenic
Bacteroides fragilis (ETBF), increased in the
mucosa of later staged CRC.Recently, the others of
our study suggest that the appearance of
Bifidobacterium as one of the indicators of
detections for colorectal cancer (Yusuf et al, 2016).
The microbiota metabolises non-digestible
food constituents into short-chain fatty acids (SCFA)
that have extensive immunological and regulatory
functions and appear to be the link in the host-
microbe interactions Within gut microbiota, several
distinct bacterial communities live at a certain ratio
under steady state condition ( Farup et al, 2016). The
level of SCFA content in faecal samples have been
shown to be related with some diseases such as IBD,
irritable bowel syndrome (IBS), cardiovascular
disease (CVD), diarrhoea, and cancer (Faujan et al,
2010). There is significant association between
levels of SCFAs and composition of the microbiota,
with high luminal concentrations resultant of
fermentation lowering colonic pH (5.5–6.5 in
proximal colon where fermentation is highest,
compared to pH 6.5–7.0 in the distal colon) and
inhibit growth of Gram-negative Enterobacteriaceae
including familiar pathogens Salmonella spp. and
Escherichia coli (Simpson and Campbell, 2015).
Therefore, a greater increase in SCFA production
and potentially a greater delivery of SCFA,
specifically butyrate, to the distal colon may result in
a protective effect (McOrist et al, 2011)
Fecal levels of butyrate in patients with
colonic neoplasia have been investigated by
different studies. Vernia et al, 1995. compared 20
patients with colorectal cancer, 8 patients colon
polyps, and healthy controls. No significant
differences were found, although patients with rectal
cancer showed slightly lower levels of propionate
and butyrate than those with more proximal cancer.
Contrast with the previous study, in our study we
found that butyrate was the best properties
diagnostic for CRC with AUC (0.84) higher than
acetate (0.71) and propionate (0.75).
The results of the present study are limited
by the relatively low number of participants and a
larger study population would provide enhanced
statistical reliability. In addition, we did not study
Butyrate Acid as a Potential Marker for Diversity of Gut Microbiota in Colorectal Cancer Patients
29

for identifies bacteria as far as we know, SCFA is
the main products of anaerobic microbial
fermentation. Another limitation is the fact that we
cannot eliminate environment factors such as diet
and everything related to microbiota.
5 CONCLUSIONS
In conclusion, this study was the first report
demonstrating of the level butyrate acids as useful
biomarkers to detect the presence of cancerous
lesions. Because the study had an exploratory design
and limited number of participants, these results
need for more validation study.
REFERENCES
Iffrig K and Weinber D., 2009. Epidemiology of
colorectal cancer, Kim EK (de), in early detection and
prevention of colorectal cancer. Slack Incorporated. 3-
18.
Ginsberg GM, Lim SS, Lauer JA, Johns BP, Sepulveda
CR., 2010. Prevention, screening and treatment of
colorectal cancer: a global and regional generalized
cost effectiveness analysis. Cost Effectiveness and
Resource Allocation. 8(2):1-16.
Sudoyo AW, Hernowo B, Krisnuhoni E, Reksodiputro
AH, Hardjodisastro D, et al., 2010 Colorectal cancer
among young native Indonesians: A
clinicopathological and molecular assessment on
microsatellite instability. Med J Indones. 199(4): 245-
251.
Sekirov I, Shannon L, Russel, Caetano M, Antunes, et al.,
2010. . Gut microbiota in health and disease. Physiol
Rev. 90: 859-904.
Faujan N.H, Abdulamir A.S, Fatimah A.B, Anas M,
Shuhaimi M, et al., 2010. The Impact of the Level of
the Intestinal Short Chain Fatty Acids in Inflammatory
Bowel Disease Patients Versus Healthy Subjects. The
Open Biochemistry Journal. 4:53-58.
Topping D.L and Clifton P.M., 2001. Short chain fatty
acids and human colonic function: roles of resistant
starch and nonstarch polysaccharides. Physiol Rev.
81;1031-1064.
Rose DJ, DeMeo MT, Kesshavarian A, and Hamaker BR.,
2007. Influence of dietary fiber on inflammatory
bowel disease and colon cancer: importance of
fermentation pattern. Nutr Rev. 65:51-62.
Vinolo M.A, Rodrigues H.G, Nachbar R.T and Curi R..,
2011. Regulation of Inflammation by Short Chain
Fatty Acids. Nutrients. 3: 858-876.
Simpson H.L and Campbell B.J., 2015. Dietary fibre–
microbiota interactions. Aliment Pharmacol Ther. 42:
158–179.
Chen HM, Yu YN, Wang JL, Lin YW, Kong X, et al.,
2013. Decreased dietary fiber intake and structural
alteration of gut microbiota in patients with advanced
colorectal adenoma. Am J Clin Nutr. 97:1044–52.
Tangerman A, Nagengast FM., 1996. A Gas
Chromatographic Analysis of Fecal Short-Chain Fatty
Acids, Using the Direct Injection Method. Anal
Biochem. 236: 1–8.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al.,
2011. Global cancer statistics. CA Cancer J Clin.
61:69–90.
Ji BT, Devesa SS, Chow WH, Jin F, Gao YT,. 1998.
Colorectal cancer incidence trends by subsite in urban
Shanghai, 1972-1994. Cancer Epidemiol Biomarkers
Prev. 7:661–6.
Wei Z, Cao S, Liu S, Yao Z, Sun T, et al., 2016. Could gut
microbiota serve as prognostic biomarker associated
with colorectal cancer patients’ survival? A pilot study
on relevant mechanism. Oncotarget, 7 (29): 158-172.
Flanagan L, Schmid J, Ebert M, Soucek P, Kunicka T,et
al., 2014. Fusobacterium nucleatum associates with
stages of colorectal neoplasia development, colorectal
cancer and disease outcome. Eur J Clin Microbiol
Infect Dis. 33:1381-1390.
Boleij A, Hechenbleikner EM, Goodwin AC, Badani R,
Stein EM,et al., 2015. The Bacteroides fragilis toxin
gene is prevalent in the colon mucosa of colorectal
cancer patients. Clin Infect Dis. 60:208-215.
Yusuf F, Ilyas S, Damanik HA, Fatchiyah F., 2016.
Microbiota Composition, HSP70 and Caspase 3
Expression as Marker for Colorectal Cancer Patients
in Aceh, Indonesia. Acta Medica Indonesiana. 48(4):
289-299.
Farup PG, Rudi K and Hested K., 2016. Faecal
short-chain fatty acids - a diagnostic biomarker
for irritable bowel syndrome?. BMC
Gastroenterology. 16:51.
Simpson H.L and Campbell B.J., 2015. Dietary
fibre–microbiota interactions. Aliment
Pharmacol Ther. 42: 158–179.
McOrist AL, Miller RB, Bird AR, Keogh JB, et al.,
2011. Fecal Butyrate Levels Vary Widely
among Individuals but Are Usually Increased by
a Diet High in Resistant Starch. J. Nutr. 141:
883–889.
Vernia P. and Cittadini M., 1995. Short chain fatty
acids and colorectal cancer. Eur J Clin Nutr
1995;49:18--20.
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
30
