
The Level of Zinc Serum after Oral Zinc on Mice with Escherichia
Coli LPS- Induced Diarrhea
Sulaiman Yusuf
1*
, Yati Soenarto
2
, Muhammad Juffrie
2
, Wiryatun Lestariana
3
, Mudatsir
4
1
Department of Paediatric,
M
edical Faculty, Syiah
K
uala University, Banda Aceh/d
r
. ZainoelAbidin Hospital,
Aceh, Indonesia
2
Department of Paediatric, Medical Faculty, Gadjah Mada University,Yogyakarta, Indonesia
3
Department of Biochemistry, Medical Faculty, Gadjah Mada University,Yogyakarta, Indonesia
4
Department of Microbiology Medical Faculty Syiah Kuala University, Aceh, Indonesia
Keywords: Zinc, Escherichia Coli, Diarrhea
Abstract: The level of zinc requirements will increase due to infection, for formation of immune function and new
cells. The advantage of Zinc is maintain the integrity of the intestinal mucosa through its function in cell
regeneration and membrane stability. The aim of this study is to determine the level of zinc serum after oral
zinc on mice diarrheal induce E.Coli-LPS. This study used a controlled trial experimental design in the
laboratory. Sample of 20 mice were randomly divided into 4 groups:1). Control group was given standard
foods, 2). Trial group was given E.Coli-LPS 2.5 mg/kg/oral once on day-1, 3). Preventive group was given
E.Coli-LPS once 2.5 mg/kg/oral once on day-1 + 30 mg/kg/oral of zinc once daily for 12 days. 4). Therapy
group was given E.Coli-LPS 2.5 mg/kg/oral once on day-1, if diarrheal was given 30 mg/kg/oral of zinc
once daily for 12 days. Blood samples of mice were taken through the orbital sinus on the 0, 5
th
, 10
th
hour,
4
th
, 8
th
and 12
th
day. Data are presented in tables and graphic. We found that higher levels of zinc in the
preventive and therapy group especially on 4
th
, 8
th
and 12
th
day. I conclusion, oral administration of zinc
increase serum zinc levels, especially in control and experimental groups.
1 INTRODUCTION
Zinc deficiency is still common, especially in
developing countries. This can be related to lack of
intake, increasing needs, and the amount of zinc loss
from the body due to diseases, especially infections.
There is associated between infection and zinc
deficiency which influence each other. Zinc
requirements of the body will increase during
infection, formation of immune functions and new
cells. Zinc deficiency can cause suppression of
immune function, making it easier for infection to
occur (Baqui, 2006; King, 2003).
Zinc plays a role in maintaining the integrity of
the intestinal mucosa through its function in cell
regeneration and membrane stability. Zinc has a
direct impact on intestinal villi, disaccharidase brush
border activity and intestinal water and electrolyte
transport. Zinc also plays a role in T cell function
and enhances immunity thereby reducing the
severity of diarrhea (Roy, 1992).
The risk of zinc deficiency in Indonesia is
estimated being greater because of Indonesian
community menu, especially in the low socio-
economic group, with lower animal protein
consumption even though this type of protein
contains a lot of zinc. Moreover, the menu of
Indonesian society is relatively high in phytate and
fiber in inhibits zinc absorption, such as the habit of
drinking tea every day, even on certain social groups
that consume thick tea. Moreover, they also
consume lots of beans and cereal, including the
processed products. This food contains a lot of
phytate or tannin so that the potential lack of zinc in
Indonesian society is quite higher because the
absorption of zinc will be disrupted (Nona, 2010).
Escherichia coli is the main occupant of healthy
colon flora, but also cause various diseases such as
diarrhea by releasing endotoxins, which triggers the
release of pro-inflammatory mediators.
Lipopolysaccharide (LPS) is the main component of
the cell wall of gram-negative bacteria which is also
called endotoxin and is known to be the trigger of
Yusuf, S., Soenarto, Y., Juffrie, M., Lestariana, W. and Mudatsir, .
The Level of Zinc Serum after Oral Zinc on Mice with Escherichia Coli LPS- Induced Diarrhea.
DOI: 10.5220/0008788201630167
In Proceedings of the 2nd Syiah Kuala International Conference on Medicine and Health Sciences (SKIC-MHS 2018), pages 163-167
ISBN: 978-989-758-438-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
163

several types of inflammatory or infectious reactions
in macrophage cells and other cells that have CD14
receptors. In fact, endotoxin (LPS) of bacteria that
binds to TLR (Toll Like Receptor) in DC (Dendritic
Cells) can stimulate monocyte and macrophage cells
to secrete nitric oxide (NO) and inflammatory
substances (cytokines) such as Tumor Necrosis
Factor-alpha (TNF-α) and interleukin-1-beta (IL 1 -
β), IL-6 and IL-8 (Rahman, et al., 2007). As a result
of the release of excessive pro-inflammatory
cytokines can cause symptoms of decreased blood
pressure, fever and diarrhea (Baratawidjaya, 2009).
In experimental studies, zinc deficiency has a
direct effect on the digestive tract, in the form of
villous atrophy, a decrease of disaccharidase enzyme
activity in brush borders, and impaired intestinal
transport. However, the exact mechanism that
connects the pathophysiology of diarrhea with zinc
deficiency has not been agreed yet. However, the
incidence of persistent diarrhea has been reduced by
zinc supplementation, and administration of ORS
(oral rehydration solutions) with zinc has
substantially reduced the duration and severity of
diarrhea in children with acute and persistent
diarrhea (Bhandari et al., 2002). Although the
mechanism of zinc supplementation reduces diarrhea
is unknown, zinc therapy in diarrhea patients has
shown better absorption of water and electrolytes by
the intestine, faster regeneration of intestinal
epithelial cells, increased levels of enzymes from
brush border, and increased immune response so that
it can eliminate pathogens in the intestine (Fenwick
et al., 2004).
The purpose of this study was to determine
serum zinc levels after administration of oral zinc in
mice diarrheal induced E. coli-LPS.
2 METHODS
This study is designed with Controlled Trial Design,
and done in The Laboratory of Nutrition and Food
Centre (PSPG) in GadjahMada University
Yogyakarta. Sample consist of 20 white mice
Sprague Dawleywhich is randomly chosen and
divided into 4 groups randomly and each group
consist of 5 mice. 1). Control Group (G1), was
given standard nutrition, 2). Trial Group (G2) was
given LPS E.Coli 2,5 mg/kg/oral in day 1-3, 3).
Preventive Group (G3) was given LPS E.Coli 2,5
mg/kg/oral in day 1 + zinc 30 mg/kg/oral since day 1
until 12 days, 4). Therapy Group (G4)was given
LPS E.Coli 2,5 mg/kg/oral in day 1, if diarrhea
occur, this group will be given zinc 30 mg/kg/oral
since day 1 until 12 days. Sample of the mice blood
was taken from sinus orbitalin 0, 5
th
, 10
th
hour, 4
th
,
8
th
, and 12
th
day to measure zinc level in serum
(Madiono, et, al., 2002).
3 RESULTS
The characteristics of subjects in this study include
weight and Hemoglobin level (Hb) presented in
Table 1.
Table 1. The Characteristics of subjects.
Group Mean of
Weight(gram)
Standard
of
Deviation
p-
value*
G1
G2
G3
G4
73,4
88,4
93,8
89,6
8,02
10,92
15,06
7,335
0,04
Group Mean of
Hb(g%)
Standard
of
Deviation
p-
value*
G1
G2
G3
G4
11,88
11,76
11,36
11,57
0,362
0,295
0,268
0,441
0,138
* ANOVA, p < 0,05
G1= Control group G3=Preventive groupG2= Trial
groupG4= Therapy group
3.1 The Level of Zinc based on
Treatment Time
The results of zinc level can be seen in Table 2 and
Picture 1. In control group (G1) there is no
difference of zinc level based on treatment time in 5
mice that has been evaluated, the level range is 1,14
until 1,2. However, zinc level in trial group (G2)
seems to be lower compared to group G1, G3 and
G4 which can be seen obviously in Picture 1, since
treatment time in 4
th
,8
th
and 12
th
day in group G2,
mice 3
rd
,4
th
and 5
th
experience diarrhea.
The difference also can bee seen in preventive
group (G3), zinc level in 5
th
hour and 10
th
hour after
treatment with LPS is 0,94 µg/dl and 0,77 µg/dl,
meanwhile in 4
th
, 8
th
and 12
th
day, zinc level is
higher compared to group G2 and can be seen
obviously in Picture 1, this event occur in group G3
mice 3
rd
,4
th
and 5
th
. In preventive group all mice
were given zinc everyday.
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
164
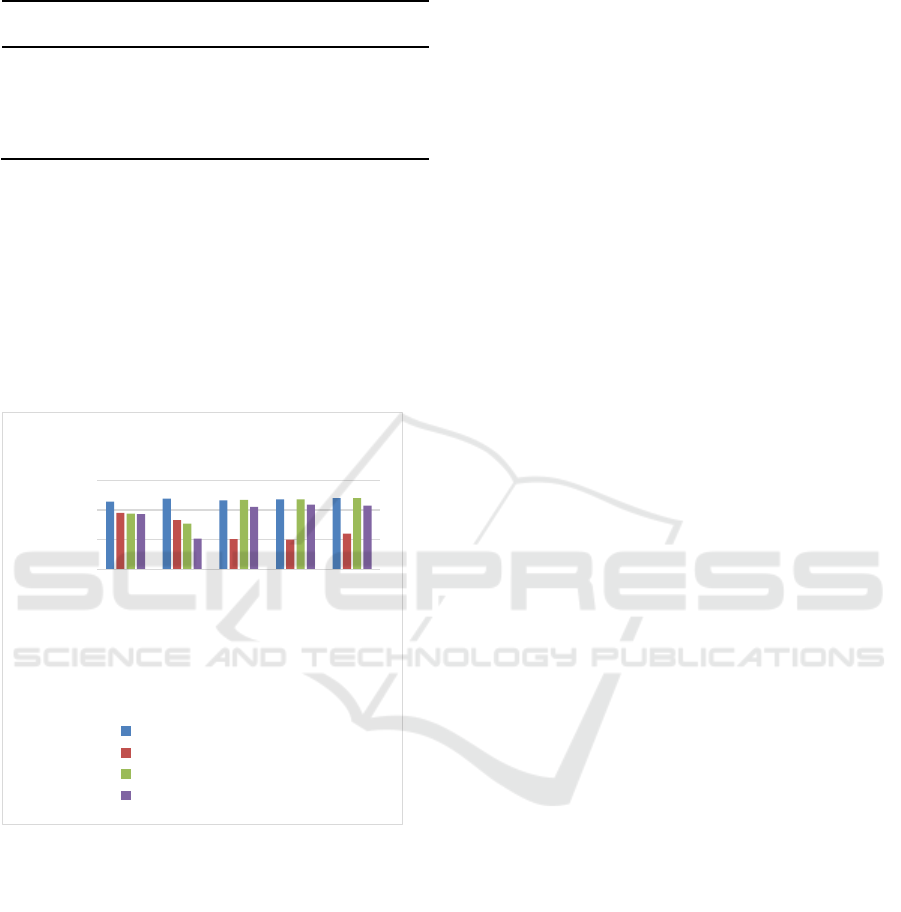
Table 2: The level of zinc (µg/dl) based on treatment time.
Treatment
time
G1
(µg/dl)
G2
(µg/dl)
G3
(µg/dl)
G4
(µg/dl)
5
th
hour 1,14 0,95 0,94 0,93
10
th
hour 1,19 0,83 0,77 0,52
4
th
day 1,16 0,51 1,17 1,05
8
th
day 1,18 0,5 1,18 1,09
12
th
day 1,2 0,6 1,2 1,07
It is the same in therapy group (G4) zinc level is
higher in mice 3
rd
, 4
th
and 5
th
compared to mice in
group G2 in 4
th
, 8
th
and 12
th
day. Actually zinc level
is higher in mice 3 in the group G3 3 days after
receiving zinc, it is also the same for mice 4 in group
G4, zinc level is higher in day 6 after receiving zinc,
mean while in mice 5 in group G4, zinc level is
higher after 10 days receiving zinc, can be seen in
Table 2 and Figure 1.
Figure 1. Diagram of zinc level in serum on control group
(G1), Trial group (G2), Preventive Group (G3) and
Therapy group (G4).
The result of zinc level in trial group (G2) is lower
than control group (G1) since 5
th
hour after
treatment time of LPS and lower in 10
th
hour, 4
th
, 8
th
and 12
th
day can be seen in Table 2 and Picture 1.
The zinc level is lower in control group (G2)
compared to preventive group (G3) and therapy
group (G4) especially in 4
th
, 8
th
and 12
th
day. Mice in
preventive and therapy group (G4) is given zinc
everyday, meanwhile in trial group (G2) is not given
zinc.
4 DISCUSSION
In Figure 1, generally we can see zinc level in
control group (G2) is lower in mice experienced
diarrhea and zinc level is higher in group received
zinc, preventive group (G3) and therapy group (G4).
In this study, mice in trial group (G2) were not given
zinc and experienced diarrhea since 3
rd
day until
12
th
day, in which zinc level is the lowest since 4
th
,
8
th
and 12
th
day. Zinc associated with gut villi
regeneration and function, therefore it will influence
formation of disaccharide enzyme such as lactase,
sucrose and maltase. Therefore, zinc can influence
process of osmotic diarrhea, most of which caused
by malabsorption andmaldigestion. In diarrhea, there
is excessive loss of zinc. Prolonged diarhhea cause
decreased the serum level of zink. There is a
circulation between diarrhea, zinc deficiency,
duration of diarrhea, and malnutrition.
Administration of micro zinc in oral can replace loss
of zinc in diarrhea (Artana et al., 2005).
Some studies show the role of zinc associated
with red blood cell formation. In biosynthesis of
heme, enzyme δ ALA dehydratase which is depend
on zinc plays an important role. There are lots of
studies show lower level of zinc can distract
synthesis of Heme. Meanwhile, the higher
supplement of zinc can also distract the absorption
of copper and iron. This can distract the immune
system response which lead to anemia (Olivares et
al., 2007). Another mechanism which shows by
another researchers were animal study with zinc
deficiency shows that decrease of erythrocyte
precursor in bone marrow and erythropoietin level in
plasma in which experiment were mouse and rat
(King et al., 2001; King et al., 2005; Konomi, 2005).
Another opinion shows that deficiency of
mineral can reduce the red blood cell lifetime
because zinc is a cofactor of Superoxide Dismutase
(SOD) in red blood cell (RBC-SOD) which
contribute in protecting from oxidative stress and
cell integrity (Powell, 2000; O’Dell, 2000).
According to the theory which developed by
Shankar & Prasad that zinc deficiency make less
productivity and activity of SOD enzyme and can
reduced free radical activity, which lead to excessive
fat peroxidation. The free radical in intestinal
mucosa cause atrophy of intestinal mucosa through
cell apoptosis. The atrophy intestinal mucosa that
caused by zinc deficiency can make decreasing
productivity and activity of SOD enzyme in
intestinal mucosa cell, therefore fre radical activity
increase which can lead to fragmentation of DNS
and also can lead to cell apoptosis. The apoptosis of
0
0,5
1
1,5
5th
hour
10th
hour
4thday8thday 12th
day
Zinc(µg/dl)
TreatmentTime
The level of zinc serum
Controlgroup(G1)
Trialgroup(G2)
Preventivegroup(G3)
Therapygroup(G4)
The Level of Zinc Serum after Oral Zinc on Mice with Escherichia Coli LPS- Induced Diarrhea
165

cell make atrophy of gut villus. Cumulative
effect of intestinal atrophy and broken of tight
junction caused the permeability of membrane
increase and disturb intestinal absorption and lead to
diarrhea (Shankar, 1998).
Patel et al in their meta-analysis found that zinc
supplementation has a modest beneficial association
(9% reduction) with incidence of diarrhea, a stronger
beneficial association (19% reduction) with
prevalence of diarrhoea and occurrence of multiple
diarrheal episodes (28% reduction) (Kalavakuri,
2017).
Zinc is usually given as zinc sulphate, zinc
acetate, or zinc gluconate, which are all water-
soluble compounds. The World Health Organization
(WHO) and the United Nations Children’s Fund
(UNICEF) recommend 10 mg to 20 mg of zinc per
day for children with diarrhoea. There are several
mechanism of action of zinc on acute diarrhoea,
some of which are specific to the gastrointestinal
system: zinc restores mucosal barrier integrity and
enterocyte brush-border enzyme activity, it promotes
the production of antibodies and circulating
lymphocytes against intestinal pathogens, and has a
direct effect on ion channels, acting as a potassium
channel blocker of adenosine 3-5-cyclic
monophosphate-mediated chlorine secretion
(Lazzerini, 2016).
The conclusion of this study are the level serum
of zinc can increase in preventive group (G3) and
therapy group (G4) after receiving oral zinc and
lower in trial control (G2) which did not received
oral zinc in mice with diarrhea induced by LPS
from E. coli.
ACKNOWLEDGEMENTS
Our thank you note is given to Mr.Yulianto as a staff
of Centre of Biotechnology Laboratory Among
University (PAU) Gadjah Mada University,
Yogyakarta which has been helpful in doing
research in trial animals and Mr.Munawar, M. App.
Stats. As a Lecturer in Faculty of Mathematics and
Science in Syiah Kuala University who has been
helping analysing the study data in statistics.
REFERENCES
Artana WD, Suraatmaja S, Aryasa KN, Suandi IKG. 2005.
Peransuplementasi mineral mikro seng terhadap
kesembuhan diare. Sari pediatri:15-8.
Baqui HA, Black RE, Walker CLF, Arifeen S, Zaman K.
2006. Zinc supplementation and serum zinc during
diarrhea. Indian J Pediatr; 73 (6) : 493-7.
Baratawidjaja KG, Rengganis I. Sitokin. 2009. Dalam:
Imunologi dasar. Edisi ke-8. Balai Penerbit FKUI.
Jakarta. h. 219-55.
Bhandari N, Bahl R, Taneja S, Strand T, Molbak K, Ulvik
RJ. 2002. Substantial reduction in severe diarrheal
morbidity by daily zinc supplementation in young
North Indian children. Pediatr;109(6).
Fenwick PK, et al. 2004. Zinc deprivation and zinc
repletion: effect on the response of rats to infection
with strongyloidesratti. Am J ClinNutr; 52:173-7.
Kalavakuri NR, Sushma Nalisetty. 2017. Serum zinc level
and effect of zinc and vitamin A supplementation in
children with diarrhea: a randomized control study. Int
J Contemp Pediatr. Jul;4(4):1501-150
King JC. 2003. Specific nutrient requirements. In:
Gershwin ME, German JB, Keen CL ed. Nutrition and
immunology principles and practice. New Jersey:
Humana Press Inc. h. 65-73.
King, LE; Fraker PJ. 2002. Zinc deficiency in mice alters
myelopoiesis and haematopoiesis. JNutr.132:3301–7.
King, LE; Frentzel JW; Mann JJ; Fraker PJ. 2005. Chronic
zinc deficiency in mice disrupted T cell lymphopoiesis
and erythropoiesis while B cell lymphopoiesis and
myelopoiesis were maintained. J Am ClinNutr.
24:494–502.
Konomi A, Yokoi K. 2005. Zinc deficiency decreases
plasma erythropoietin concentration in rats. Biol Trace
Elem Res.107:289–92.
Lazzerini M, Wanzira H. 2016. Oral zinc for treating
diarrhea in children. Cochrane Database of Systematic
Review: 12:1-2
Madiono B, Moeslichan S, Sastroasmoro S, Budiman I,
Purwanto SH. 2002.Perkiraanbesarsampel. Dalam:
Sastroasmoro S, Ismael S. (Ed). Dasar-dasar
metodologi penelitian klinis. Edisi ke-2. CV
SagungSeto. Jakarta. h. 259-87.
Nona FS. 2010. Analisis kadar besi (Fe), seng (Zn), dan
mangan (Mn). Retrieved
from:http://repository.usu.ac.id/bitstream/123456789/
19341/4/Chapter%20II.pdf. on October 12
th
, 2010.
O’Dell, BL. 2000. Role of zinc in plasma membrane
function. J Nutr. 130:1432S–6S.
Olivares, M; E Hertrampf; R Uauy. 2007. Copper and zinc
interactions in anemia: a public health perspective. In:
Kraemer K ZM, ed. Nutritional anemia. Basel,
Switzerland: Sight and LifePress.
Powell, SR. 2000. The antioxidant properties of zink. J
Nutr. 130:1447S-54S.
Rahman DY, Aulanni’am, Ranuh R. 2007. Pengaruh
pemberian probiotik Lactobacillus plantarum terhadap
penurunan kadar radikal bebas dan profil protein
penyusun brush border intestin akibat paparan LPS
dari E.coli (Pre-eliminary studi). Universitas
Brawijaya, Malang, Universitas Airlangga, Surabaya.
Roy SK, Behrens RH, Haider R, Akramuzzaman SM,
Mahalanabis D, Wahed MA, et al., 1992. Impact of
zinc supplementation on intestinal permeability in
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
166

Bangladeshi children with acute diarrhea and
persistent diarrhea syndrome. J
PediatrGastroenterolNutr; 15:289-96.
Shankar AH, Prasad AS. 1998. Zinc and immune function:
the biological basis of altered resistance to infection in
Am J ClinNutr: 447S-457S.
The Level of Zinc Serum after Oral Zinc on Mice with Escherichia Coli LPS- Induced Diarrhea
167
