
Malaria in Burkina Faso from 2000 to 2019: Assessment of Diagnostic
Tools
Silvere Dieudonne Zaongo
1,4,
*, Wilfried Noel Sam
1
, Blaise Raogo Ouedraogo
2
,
Jean-Bosco Ouedraogo
3
, Dar-Der Ji
5
1
Tianjin Medical University, Tianjin, China
2
Kunming Institute of zoology, University of Chinese Academy of Sciences, China
3
Institut de Recherche en Sciences de la Santé, Burkina Faso
4
Nankai University Second People’s Hospital, School of Medicine, Nankai University, Tianjin, China
5
International Health Program, National Yang Ming University, Taiwan
Keywords: Burkina Faso, Sub-microscopic malaria, PCR, Microscopy, Rapid Diagnostic tests
Abstract: Malaria elimination depends on the potency of surveillance tools. We assessed the efficacy of rapid
diagnostic tests (RDTs) and polymerase chain reaction (PCR) in sub-microscopic malaria detection. Cross
sectional or screening studies realized in Burkina Faso from 2000 to 2019 were found in PubMed using
keywords “malaria”, “PCR” and “Burkina Faso” and specific inclusion criteria. Malaria prevalence and sub-
microscopic (SM) were calculated from PCR, LM, and RDTs results. Overall, 6 studies (4 in Nanoro, 1 in
Bourasso, and 1 in Bobo-Dioulasso) fit the inclusion criteria. The prevalence by PCR in Bourasso (before
2009) was the highest compared to Nanoro (92% vs 27.3%, p<0.001) and Bobo-Dioulasso (92% vs 34.5%,
p<0.001). From PCR results it seems that SM prevalence is relatively stable over the last 20 years
independently from the location (11.4% in Bourasso, 10.3% in Nanoro, 19.9% in Bobo-Dioulasso). Except
in Nanoro where SM
HRP2
was higher than SM
PCR
(12.8% vs 10.3%, p=0.04), RDT HRP2 and RDT pLDH
failed to compete with PCR in SM detection. Although implementation of RDTs have triggered the
reduction of malaria cases, they are not suitable for sub-microscopic malaria detection. Therefore, novel
diagnostic tools as sensitive as PCR and as easy to perform as RTDs are needed.
1 INTRODUCTION
From 2010 to 2015, the number of infected and
death cases of malaria have reduced by 21% and
29% among all age groups. Despite that, malaria is
still a public health issue especially in Sub-Saharan
Africa. This region is the most affected where
Children under 5 years and pregnant women are the
most vulnerable population in terms of mortality and
morbidity (UNICEF and WHO, 2000). An early
diagnostic of malaria is essential to prevent the fatal
outcomes such as anemia, low bird weight, and
mother and/or child death (Steketee et al., 1996;
Luxemburger et al., 1997). In 2015, 90% of cases
and 92% of malaria deaths were reported in the same
African region although the death rate felt by 35%
among children under 5 years. Recent estimations
suggest that 91 countries and areas had ongoing
malaria transmission (WHO, 2016).
In Burkina Faso, malaria represents 63.2% of
hospitalizations and 49.6% of deaths among children
under 5 year-old. Noticing the reduction of malaria
cases worldwide, the National Malaria Control
Program of Burkina Faso aims to end the disease by
2030. Thus, political commitment, implementation
of Artemisinin Combination Therapy (ACT), better
access of population to diagnostic and vector control
strategies (insecticide treated bed nets) are already
implemented ((PNLP), 2014). However, the success
of malaria surveillance depends on the performance
of existing surveillance tools (Breman and
Holloway, 2007) especially on asymptomatic
individuals who can exhibit low-density malaria
infection or submicroscopic malaria (Cheng et al.,
2015). The major tools so far used are light
microscopy (LM), rapid diagnostic tests (RDTs) and
polymerase chain reaction (PCR). Microscopy has
been the main tool for more than two decades as it is
cheap (Siala et al., 2010); RDTs recently introduced
Zaongo, S., Sam, W., Ouedraogo, B., Ouedraogo, J. and Ji, D.
Malaria in Burkina Faso from 2000 to 2019: Assessment of Diagnostic Tools.
DOI: 10.5220/0008788001490155
In Proceedings of the 2nd Syiah Kuala International Conference on Medicine and Health Sciences (SKIC-MHS 2018), pages 149-155
ISBN: 978-989-758-438-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
149

as an alternative to microscopy weakness (Makler et
al., 1998) are implemented in Burkina Faso since
2009 (Natama et al., 2017); and PCR, introduced in
malaria regions in the 1990s, showed increased
prevalence of malaria in communities screened.
Herein, we proposed to assess these malaria
diagnostic tools efficacy in sub-microscopic malaria
detection over the last 20 years in Burkina Faso.
2 MATERIALS AND METHODS
2.1 Literature Search and Inclusion
Criteria
Were considered as relevant, all articles identified in
PubMed using search terms “malaria”, “PCR” and
“Burkina Faso”. The literature research was done on
April 10
th
, 2019. The studies were eligible only if
they had the inclusion criteria that are: (a) the
articles were written in English and published within
2000 to the 4
th
of March 2019, (b) the study
participants consisted of a population sample of
individuals in, an endemic area who were not chosen
on the basis of malaria symptoms or test results, (c)
cross sectional studies or screening studies, (d) Data
of light microscopy (LM), RDTs [Histidine Rich
Protein 2 (HRP2) and/or Plasmodium Lactate
Dehydrogenase (pLDH)] and/or PCR/ quantitative
PCR (qPCR) / Retro-Transcription PCR (RT-PCR) /
direct blood PCR (db-PCR) should be presented, (e)
at least Plasmodium falciparum infection was
detected. For the screening of cohorts, only the data
at the inclusion (before any intervention) were
considered.
2.2 Malaria Prevalence by PCR, LM,
and RDTs
Based on the results of each diagnostic tool, malaria
prevalence (P) was estimated by dividing the total
number of positive individual by the total number of
screened population. Therefore, the prevalence by
PCR (P
PCR
), Microscopy (P
LM
), HPR2 (P
HRP2
), and
pLDH (P
pLDH
) were used to estimate the sub-
microscopic malaria ongoing for 20 years in Burkina
Faso.
2.3 Burkina Faso Map and Study Sites
Location
The map was drawn in RStudio software (Version
1.0.153, RStudio, Inc) using the packages “map and
mapdata”. To represent the study sites location, their
geographic coordinates were integrated before the
map generating.
2.4 Sub-microscopic Malaria
Evaluation
The sub-microscopic (SM) infection was calculated
either based on PCR or RDTs results. When the
PCR results were considered:
SM
PCR
= P
PCR
– P
LM
.
Considering the RDTs as reference:
SM
HRP2
= P
HRP2
– P
LM
; or SM
pLDH
= P
pLDH
– P
LM
.
2.5 Data Analysis
Data were entered on Microsoft Excel 2013 then
subsequently transferred and analyzed on SPSS
(Version 20, IBM Corporation). The Fisher exact
test was used to compare the proportions as
previously described (Campbell, 2007; Richardson,
2011). Therefore, the prevalence based on diagnostic
tools or location were compared using α=0.05 as
significance level.
3 RESULTS
3.1 Descriptive Statistics
From PubMed using the specific keywords, 92
articles were found and 6 studies fit the inclusion
criteria (Figure 1).
Figure 1.
Paper mining flowchart
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
150

They were predominantly conducted in Nanoro (4/6,
66.7%). Among the 2 remaining studies one was
performed in Bourasso (1/6, 16.7%) and the other in
Bobo-Dioulasso (1/6, 16.7%). The geographical
location of Nanoro (in Center-West, located at 85
km of Ouagadougou the capital city) and Bobo-
Dioulasso (in West, the second largest town of the
country) are represented in Fig 2. Bourasso is a rural
village located between Bobo-Dioulasso and
Ouagadougou; its location is closer to the Malian
border of the country (see Figure 2).
Figure 2. Burkina Faso map with study sites location
The most targeted populations were pregnant
women and infants (4/6, 66.7%) as shown in Table 1
Location Target population Positive test Total
sam
p
le
Ref
PCR Microscopy RDT
HRP2
RDT
pLDH
Nanoro Infants 16 0 3 - 400 (Natama et
al., 2017)
Bobo-
Dioulasso
Pregnant women 224 95 134 102 650 (Kyabayinze
et al., 2016)
Nanoro All inhabitants 139 122 136 - 283 (Mens et al.,
2012)
Nanoro Pregnant women 201 112 178 380 (Kattenberg
et al., 2012)
Nanoro Infants 120 62 - - 678 (Natama et
al., 2018)
Bourasso All inhabitants 185 162 - - 201 (Stich et al.,
2006)
Table 1. Details of the selected studies
3.2 Sub-microscopic Malaria
Prevalence
From the selected articles, a total of 2592 people
were screened by PCR and LM. The number was
reduced when we split the screened populations into
before 2009 (201 screened people) and after 2009
(2391 screened people). However, considering RDT
HRP2 and RDT pLDH, which were not used in
every study, the screened populations were 1713 and
650 respectively.
Overall, in 20 years the prevalence of malaria by
PCR, and microscopy was 33.1 % (885/2592), and
21.3% (553/2592). Considering that RDTs were
implemented after 2009, the Prevalence of malaria
by PCR, LM, HRP2, and pLDH from 2009 until
now is at 29.3 % (700/2391), 16.4% (391/2391),
23.3% (451/1713), and 16% (102/650). Thus, the
sub-microscopic SM
PCR,
SM
HRP2,
and
SM
pLDH
are
estimated at 12.9%, 6.9% and -0.4% (considered as
0) respectively.
In Nanoro, the malaria prevalence with PCR
(P
PCR
) was 27.3% (476/1741). It was the highest
estimated prevalence in comparison to microscopy
(P
LM
= 17%, 296/1741), and HRP2 (P
HRP2
=29.8%,
317/1063). Thus, SM
PCR
= 10.3%, and SM
HRP2
=
12.8%. In Bobo-Dioulasso, P
PCR
was at 34.5%
(224/650) whereas P
LM
(14.6% (95/650), HRP2
(P
HRP2
=20.6%, 134/650), and pLDH (P
pLDH
= 15.7%,
102/650) were showing lower prevalence. The
estimated SM
PCR
= 19.9%, SM
HRP2
=
6%, and
Malaria in Burkina Faso from 2000 to 2019: Assessment of Diagnostic Tools
151
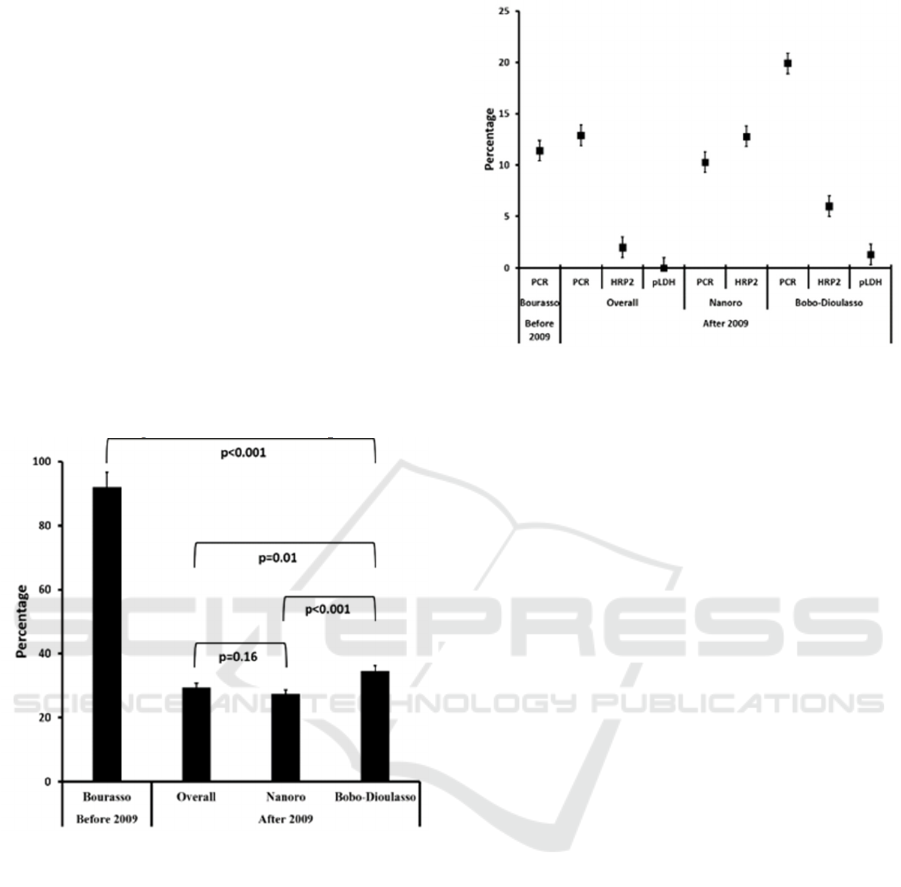
SM
pLDH
=
1.3%. In Bourasso, malaria prevalence
using PCR (P
PCR
) was estimated at 92% (185/201).
Therefore, SM
PCR
was at 11.4% as the prevalence by
microscopy (P
LM
) was 80.6% (162/201).
Interestingly, we noted from Fig. 3 that malaria
prevalence by PCR in Bourasso was the highest in
comparison to Nanoro (92% vs 27.3%, p<0.001) and
Bobo-Dioulasso (92% vs 34.5%, p<0.001). The
same observation was noted when we compared the
prevalence in Bourasso to the overall prevalence
after 2009 (92% vs 29.3%, p<0.001). Except in
Bourasso, the investigations conducted in Nanoro
and Bobo-Dioulasso were performed after 2009.
After 2009, the overall prevalence vs Nanoro (29.3
vs 27.3%, p= 0.16) was relatively similar while
overall prevalence vs Bobo-Dioulasso (29.3% vs
34.5%, p=0.01) was statistically different. Moreover,
compared to Nanoro the prevalence in Bobo-
Dioulasso was higher (34.5% vs 27.3%, p<0.001).
Figure 3. Prevalence by PCR before and after the
implementation of RDTs in Burkina Faso
In comparing the SM calculated from PCR and
RDTs, we noted that PCR had the highest detection
rate. Moreover, considering PCR results it seems
that SM is relatively stable over the last 20 years
independently from the location (11.4% in Bourasso,
10.3% in Nanoro, 19.9% in Bobo-Dioulasso, and
12.9% overall). Except in Nanoro where SM
HRP2
was higher than SM
PCR
(12.8% vs 10.3%, p=0.04)
the general trend is showing that RDT HRP2 failed
to compete with PCR in terms of SM diagnostic.
However, RDT HRP2 showed a relatively higher
sensitivity for SM diagnostic compared to RDT
pLDH (6.9% vs 0% Overall, p= and 12.8 vs 1.3 in
Bobo-Dioulasso). The details of the aforementioned
results are presented in Figure 4.
Figure 4. Submicroscopic malaria prevalence based on
PCR, RDTs, locations and period.
4 DISCUSSION
From PubMed, few articles fitting the selection
criteria were found. 3 regions were of interest:
Bourasso (before 2009), Nanoro and Bobo-
Dioulasso (after 2009) (Figure 1 and 2). Bourasso is
a village belonging to the rural area located near the
Malian border. Like Bobo-Dioulasso, malaria
transmission in Bourasso is holoendemic with a peak
during the rainy season May to October (Soma et al.,
2018). Thus, the prevalence in Bourasso before 2009
could be assimilated to the one observed in Bobo-
dioulasso during the same period. Nanoro and Bobo-
Dioulasso are well-known for their health research
centers of reference. That is probably why most of
the studies were conducted in Nanoro (Figure 1)
which in contrary is a hyperendemic transmission
area from July to November (Natama et al., 2018).
We found that prevalence by PCR in Bourasso
(before 2009) was the highest (92%). After 2009,
the prevalence was estimated at 27.3% in Nanoro
and 34.5% in Bobo-Dioulasso. From 92% the
prevalence was reduced to 29.3% (Overall) after
2009 (Figure 3). This suggest that after the usage of
RDTs, malaria cases were somehow divided by 3.
This is parallel with earlier studies addressing the
positive impact of RDT as they enhanced the
surveillance for malaria elimination (Linn et al.,
2015; Donald et al., 2016). However, malaria
elimination program also depends on the ability of
diagnostic tools to detect SM.
We evaluated the SM rates by PCR, and RDT.
Considering PCR results it seems that SM is
relatively stable over the last 20 years independently
from the study site (between 10.3% and 19.9%, see
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
152

figure 4). In contrary, RDTs HRP2 and pLDH failed
to detect SM. This could be explained by the high
sensitivity of PCR which is able to detect very low
levels of parasitemia (de Monbrison et al., 2003);
while (a) Antigen-based detection RDTs are not
efficient for the infection detection during the early
stages (Siala et al., 2010), (b) their problems of
storage are well-known (Gamboa et al., 2010; WHO
et al., 2011) and (c) they are not quantitative enough
to distinguish between levels of infections (Murray
and Bennett, 2009). Golassa and coll. after
comparing PCR, and RDT had concluded that only
PCR was able to detect low-density malaria
infection also called asymptomatic malaria (Golassa
et al., 2013). In areas where PCR cannot be
performed daily, this finding represents a threat to
elimination programs. Actually, SM infections only
rarely provoke acute diseases (Rogier et al., 1996),
but they are capable of infecting mosquitoes and
continuing the transmission (Muirhead-Thomson,
1957; Coleman et al., 2004). Moreover, it is known
that SM can persist for several months without any
symptoms that would prompt treatment seeking
(Roper et al., 1996); which is not appropriate for
pregnant women in high transmission areas as they
are exposed to anemia, placental malaria, and low-
birth weight (Steketee et al., 1996). Considering our
findings suggesting there are lot of SM ongoing in
Burkina Faso, and knowing the risks of this form of
malaria, we could therefore assume that it is an urge
to promote more sensitive diagnostic tests.
Plasmodium feeds on hemoglobin and excrete
iron under toxic form for the parasite that converts it
into hemozoin. Hemozoin behaves like little
magnets that are detectable and measurable. In the
past few years, Magnets detection technique is
becoming prominent (Kim et al., 2010; Mens et al.,
2010; Castilho Mde et al., 2011; Yuen and Liu,
2012). From research published, RDTs limit of
detection was estimated at 100 parasites/ul while the
threshold of the device detecting hemozoin was
equivalent to ≤ 30 parasites/ul (Butykai et al., 2013).
The technic is faster than RDT and more than 3
times as accurate as current kits. To our knowledge,
despite Sub-Saharan African countries like Burkina
Faso are majorly exposed to malaria, the magnet
detection technique has not been used yet.
CONFLICT OF INTERESTS
The authors declare that they have no competing
interests
ACKNOWLEDGEMENTS
Thanks to N. Aida N. Ouedraogo for her help with
the proofreading of this paper.
REFERENCES
Programme National de Lutte contre le Paludisme
(PNLP), 2014. Directives nationales de prise en charge
du paludisme - revision de mars 2014. pp. 29.
Breman, J., Holloway, C., 2007. Malaria surveillance
counts. American Journal of Tropical Medicine and
Hygiene 77: 36-47.
Butykai, A., Orban, A., Kocsis, V., Szaller, D., Bordacs,
S., Tatrai-Szekeres, E., Kiss, L., Bota, A., Vertessy,
B., Zelles, T., Kezsmarki, I., 2013. Malaria pigment
crystals as magnetic micro-rotors: key for high-
sensitivity diagnosis. Scientific Reports 3: 1431.
10.1038/srep01431.
Campbell, I., 2007. Chi-squared and Fisher-Irwin tests of
two-by-two tables with small sample
recommendations. Statistics in Medicine 26: 3661-
3675. 10.1002/sim.2832.
Castilho Mde, S., Laube, T., Yamanaka, H., Alegret, S.,
Pividori, M., 2011. Magneto immunoassays for
Plasmodium falciparum histidine-rich protein 2 related
to malaria based on magnetic nanoparticles. Analytical
Chemistry 83: 5570-5577. 10.1021/ac200573s.
Cheng, Q., Cunningham, J., Gatton, M. L., 2015.
Systematic review of sub-microscopic P. vivax
infections: prevalence and determining factors. PLOS
Neglected Tropical Diseases 9: e3413.
10.1371/journal.pntd.0003413.
Coleman, R., Kumpitak, C., Ponlawat, A., Maneechai, N.,
Phunkitchar, V., Rachapaew, N., Zollner, G.,
Sattabongkot, J., 2004. Infectivity of asymptomatic
Plasmodium-infected human populations to Anopheles
dirus mosquitoes in western Thailand. Journal of
Medical Entomology 41: 201-208.
de Monbrison, F., Angei, C., Staal, A., Kaiser, K., Picot,
S., 2003. Simultaneous identification of the four
human Plasmodium species and quantification of
Plasmodium DNA load in human blood by real-time
polymerase chain reaction. Transactions of the Royal
Society of Tropical Medicine and Hygiene 97: 387-
390.
Donald, W., Pasay, C., Guintran, J. O., Iata, H. Anderson,
K., Nausien, J., Gresty, K. J., Waters, N. C.,
Vestergaard, L. S., Taleo, G., Cheng, Q., 2016. The
Utility of Malaria Rapid Diagnostic Tests as a Tool in
Enhanced Surveillance for Malaria Elimination in
Vanuatu. PLoS One 11: e0167136.
10.1371/journal.pone.0167136.
Gamboa, D., Ho, M., Bendezu, J., Torres, K., Chiodini, P.,
Barnwell, J., Incardona, S., Perkins, M., Bell, D.,
McCarthy, J., Cheng, Q., 2010. A large proportion of
P. falciparum isolates in the Amazon region of Peru
lack pfhrp2 and pfhrp3: implications for malaria rapid
Malaria in Burkina Faso from 2000 to 2019: Assessment of Diagnostic Tools
153

diagnostic tests. PLoS One 5: e8091.
10.1371/journal.pone.0008091.
Golassa, L., Enweji, N., Erko, B., Aseffa, A., Swedberg,
G., 2013. Detection of a substantial number of sub-
microscopic Plasmodium falciparum infections by
polymerase chain reaction: a potential threat to malaria
control and diagnosis in Ethiopia. Malaria Journal 12:
352. 10.1186/1475-2875-12-352.
Kattenberg, J., Tahita, C., Versteeg, I., Tinto, H., Traore
Coulibaly, M., D'Alessandro, U., Schallig, H., Mens,
P., 2012. Evaluation of antigen detection tests,
microscopy, and polymerase chain reaction for
diagnosis of malaria in peripheral blood in
asymptomatic pregnant women in Nanoro, Burkina
Faso. American Journal of Tropical Medicine and
Hygiene 87: 251-256. 10.4269/ajtmh.2012.12-0125.
Kim, C., Wilson, E., DeRisi, J., 2010. Improved methods
for magnetic purification of malaria parasites and
haemozoin. Malaria Journal 9: 17. 10.1186/1475-
2875-9-17.
Kyabayinze, D., Zongo, I., Cunningham, J., Gatton, M.,
Angutoko, P., Ategeka, J., Compaore, Y.,
Muehlenbachs, A., Mulondo, J., Nakalembe, M.,
Some, F., Ouattara, A., Rouamba, N., Ouedraogo, J.,
Hopkins, H., Bell, D., 2016. HRP2 and pLDH-Based
Rapid Diagnostic Tests, Expert Microscopy, and PCR
for Detection of Malaria Infection during Pregnancy
and at Delivery in Areas of Varied Transmission: A
Prospective Cohort Study in Burkina Faso and
Uganda. PLoS One 11: e0156954.
10.1371/journal.pone.0156954.
Linn, A. M., Ndiaye, Y., Hennessee, I., Gaye, S., Linn, P.,
Nordstrom, K., McLaughlin, M., 2015. Reduction in
symptomatic malaria prevalence through proactive
community treatment in rural Senegal. Tropical
Medicine and International Health 20: 1438-1446.
10.1111/tmi.12564.
Luxemburger, C., Ricci, F., Nosten, F., Raimond, D.,
Bathet, S., White, N., 1997. The epidemiology of
severe malaria in an area of low transmission in
Thailand. Transactions of the Royal Society of
Tropical Medicine and Hygiene 91: 256-262.
Makler, M., Palmer, C., Ager, A., 1998. A review of
practical techniques for the diagnosis of malaria.
Annals of Tropical Medicine and Parasitology 92:
419-433.
Mens, P., de Bes, H., Sondo, P., Laochan, N.,
Keereecharoen, L., van Amerongen, A., Flint, J., Sak,
J., Proux, S., Tinto, H., Schallig, H., 2012. Direct
blood PCR in combination with nucleic acid lateral
flow immunoassay for detection of Plasmodium
species in settings where malaria is endemic. Journal
of Clinical Microbiology 50: 3520-3525.
10.1128/JCM.01426-12.
Mens, P., Matelon, R., Nour, B., Newman, D., Schallig,
H., 2010. Laboratory evaluation on the sensitivity and
specificity of a novel and rapid detection method for
malaria diagnosis based on magneto-optical
technology (MOT). Malaria Journal 9: 207.
10.1186/1475-2875-9-207.
Muirhead-Thomson, R., 1957. The malarial infectivity of
an African village population to mosquitoes
(Anopheles gambiae); a random xenodiagnostic
survey. American Journal of Tropical Medicine and
Hygiene 6: 971-979.
Murray, C., Bennett, J., 2009. Rapid Diagnosis of Malaria.
Interdisciplinary Perspectives on Infectious Diseases
2009: 415953. 10.1155/2009/415953.
Natama, H., Ouedraogo, D., Sorgho, H., Rovira-Vallbona,
E., Serra-Casas, E., Some, M., Coulibaly-Traore, M.,
Mens, P., Kestens, L., Tinto, H., Rosanas-Urgell, A.,
2017. Diagnosing congenital malaria in a high-
transmission setting: clinical relevance and usefulness
of P. falciparum HRP2-based testing. Scientific
Reports 7: 2080. 10.1038/s41598-017-02173-6.
Natama, H., Rovira-Vallbona, E., Some, M., Zango, S.,
Sorgho, H., Guetens, P., Coulibaly-Traore, M., Valea,
I., Mens, P., Schallig, H., Kestens, L., Tinto, H.,
Rosanas-Urgell, A., 2018. Malaria incidence and
prevalence during the first year of life in Nanoro,
Burkina Faso: a birth-cohort study. Malaria Journal
17: 163. 10.1186/s12936-018-2315-4.
Natama, H. M., Rovira-Vallbona, E., Some, M. A., Zango,
S. H., Sorgho, H., Guetens, P., Coulibaly-Traore, M.,
Valea, I., Mens, P. F., Schallig, H., Kestens, L., Tinto,
H., Rosanas-Urgell, A., 2018. Malaria incidence and
prevalence during the first year of life in Nanoro,
Burkina Faso: a birth-cohort study. Malaria Journal
17: 163. 10.1186/s12936-018-2315-4.
Richardson, J. T., 2011. The analysis of 2 x 2 contingency
tables--yet again. Statistics in Medicine 30: 890;
author reply 891-892. 10.1002/sim.4116.
Rogier, C., Commenges, D., Trape, J., 1996. Evidence for
an age-dependent pyrogenic threshold of Plasmodium
falciparum parasitemia in highly endemic populations.
American Journal of Tropical Medicine and Hygiene
54: 613-619.
Roper, C., Elhassan, I., Hviid, L., Giha, H., Richardson,
W., Babiker, H., Satti, G., Theander, T., Arnot, D.,
1996. Detection of very low level Plasmodium
falciparum infections using the nested polymerase
chain reaction and a reassessment of the epidemiology
of unstable malaria in Sudan. American Journal of
Tropical Medicine and Hygiene 54: 325-331.
Siala, E., Ben, A., Bouratbine, A., Aoun, K., 2010.
Actualités du diagnostic biologique du paludisme.
Revue Tunisienne d’Infectiologie 4: 5-9.
Soma, D., Kassie, D., Sanou, S., Karama, F., Ouari, A.,
Mamai, W., Ouedraogo, G., Salem, G., Dabire, R.,
Fournet, F., 2018. Uneven malaria transmission in
geographically distinct districts of Bobo-Dioulasso,
Burkina Faso. Parasites & Vectors 11: 296.
10.1186/s13071-018-2857-x.
Steketee, R., Wirima, J., Campbell, C., 1996. Developing
effective strategies for malaria prevention programs
for pregnant African women. American Journal of
Tropical Medicine and Hygiene 55: 95-100.
Stich, A., Oster, N., Abdel-Aziz, I. Z., Stieglbauer, G.,
Coulibaly, B., Wickert, H., McLean, J., Kouyate, B.
A., Becher, H., Lanzer, M., 2006. Malaria in a
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
154

holoendemic area of Burkina Faso: a cross-sectional
study. Parasitology Research 98: 596-599.
10.1007/s00436-005-0104-9.
UNICEF, World Health Organization, 2000. Malaria
prevention and treatment. The prescriber, 1-16.
World Health Organization, 2016. World malaria report
2016. 186.
World Health Organization, World Bank, Foundation for
Innovative New Diagnostics, 2011. Malaria rapid
diagnostic test performance : summary results of
WHO malaria RDTs product testing:rounds 1-3
[2008-2011]. Geneva, World Health Organization: 16.
Yuen, C., Liu, Q., 2012. Magnetic field enriched surface
enhanced resonance Raman spectroscopy for early
malaria diagnosis. Journal of Biomedical Optics 17:
017005. 10.1117/1.JBO.17.1.017005.
Malaria in Burkina Faso from 2000 to 2019: Assessment of Diagnostic Tools
155
