
Pre-stimulation of Bone-marrow Derived Eosinophils with CCL24
Alters Responses to TLR Ligands and Helminth Extracts
B. C. Buerfent
1
, A. Ehrens
1
, W. Stammin
g
e
r
1
, A. Hoerauf
1,2
, M. P. Hübne
r
1,*
1
Institute for Medical Microbiology, Immunology and Parasitology, University Hospital of Bonn, Germany
2
German Center for Infection Research (DZIF), partner site Bonn-Cologne, Bonn, Germany
Keywords: Eosinophils, Filaria, Helminth, TLR, LPS, Eotaxin
Abstract: Eosinophil granulocytes are a hallmark of helminth infection, provide protection against helminth infections
and elicit detrimental effects during allergy and asthma. Moreover, eosinophils are associated with diabetes,
arthritis and sepsis. Thus, eosinophils have a broad range of implications, contributing to homeostasis as
well as pathogenesis of various diseases. In the current study we used murine bone-marrow derived
eosinophils (bmEos) to investigate the impact of eosinophil pre-stimulation with the chemoattractants
CCL11 and CCL24 (eotaxin-1 and 2) on TLR2, TLR4 and filarial extract-induced eosinophil responses.
Generation of bmEos consistently resulted in approximately 50 million bmEos from a single donor mouse
and a purity and viability above 95%. Upon stimulation with CCL24, TLR2, TLR4, and filarial extract,
bmEos released different quantities of IL-4, IL-6, CCL5, as well as CXCL1. CCL24 pre-stimulation
partially affected those responses. Furthermore, CCL24 pre-stimulation of bmEos reduced the expression of
the eotaxin receptor CCR3 independently of TLR2 stimulation. In contrast, expression of adhesion molecule
ICAM-1 was increased by TLR2 stimulation, but not affected by CCL24 pre-stimulation. Hence, our results
reveal an impact of CCL24 on bmEos activation. bmEos present a promising tool to study eosinophil
responses that may help to further characterize their role in different immunological contexts and overcome
the limitations given by the low eosinophil frequencies present in non-helminth-infected individuals.
1 INTRODUCTION
Eosinophil granulocytes are most famous for their
involvement in the pathogenesis of allergies and
asthma (Fulkerson and Rothenberg, 2013) as well as
their characteristic expansion and protective effect
during helminth infection (Gentil et al., 2014).
However, eosinophils further support anti-bacterial
responses, contribute to metabolic homeostasis and
impact autoimmune diseases. Accordingly,
eosinophils recognize pathogen associated molecular
patterns and possess anti-bacterial functions due to
the release of bactericidal NET like structures and
phagocyte-recruiting chemokines and are discussed
as potential marker for the severity of bacterial
sepsis (Merino et al., 2012). Moreover, adipose
tissue eosinophils help to maintain glucose and
insulin tolerance by driving alternative macrophage
activation via the release of IL-4 (Wu et al., 2011).
Such a beneficial role of eosinophils was also
described during inflammatory arthritis, which was
mitigated by helminth-induced eosinophils (Chen et
al., 2016).
Eosinophil granulocytes produce and detect
numerous chemokines and cytokines and express
pattern recognition receptors including toll-like-
receptors (Rosenberg et al., 2013). In general, IL-5
is the main inducer of eosinophils and eotaxins that
bind to the chemokine receptor CCR3 direct
eosinophils to the site of inflammation. Thus,
eosinophils are involved in a broad range of
homeostatic and inflammatory conditions and
essentially modulate immune responses and
pathogenesis. We here provide evidence for the
impact of the eotaxins CCL11 and CCL24 on
subsequent TLR-induced and filarial extract-induced
immune responses of bone-marrow derived
eosinophils (bmEos), which may contribute to the
diverse spectrum of eosinophil functions.
Buerfent, B., Ehrens, A., Stamminger, W., Hoerauf, A. and Hübner, M.
Pre-stimulation of Bone-marrow Derived Eosinophils with CCL24 Alters Responses to TLR Ligands and Helminth Extracts.
DOI: 10.5220/0008787800050009
In Proceedings of the 2nd Syiah Kuala International Conference on Medicine and Health Sciences (SKIC-MHS 2018), pages 5-9
ISBN: 978-989-758-438-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reser ved
5

2 METHODS
2.1 Ethics Statement and Mice
BALB/c mice (Janvier, Saint Berthevin Cedex,
France) were housed at the Institute for Medical
Microbiology, Immunology and Parasitology of the
University Hospital Bonn, Germany, with access to
food and water ad libitum. All experiments were
approved by the Landesamt für Natur, Umwelt und
Verbraucherschutz, Cologne, Germany and
performed according to the European Union animal
welfare guidelines.
2.2 In Vitro Eosinophil Differentiation
from Bone-marrow
Eosinophils were differentiated from bone-marrow
of naïve adult mice by stimulation with 100ng/ml
stem cell factor (SCF) and FMS-like tyrosine kinase
3 ligand (FLT3L) for four days followed by culture
with 20ng/ml IL-5 for eight days (all Peprotech,
Rocky Hill, USA, Fig. 1A) (Dyer et al., 2008). Cells
were adjusted to 1x10
6
cells/mL and incubated at
37°C and 5% CO
2
. Half of the medium containing
advanced RPMI 1640, 20% heat-inactivated fetal
calf serum, 1M HEPES, 10.000 IU/mL penicillin,
10µg/mL streptomycin, 1X GlutaMAX
TM
(all
Gibco® Technologies, Waltham, USA) was
replaced every other day. Adherent cells were
removed at day 8 and eosinophil purity was checked
at day 12.
2.3 In Vitro Stimulation of
Bone-marrow Derived Eosinophils
1x10
6
bmEos were pre-stimulated for 24 hours with
100ng/mL CCL11 or CCL24 (both Peprotech,
Rocky Hill, USA) in eosinophil growth medium.
Subsequently, cells were re-stimulated for 24 h with
200ng/mL lipopolysaccharide (LPS) ultrapure,
500ng/mL Pam3CSK4 (P3C) (both InvivoGen, San
Diego, USA) or 25µg/mL Litomosoides sigmodontis
crude adult worm extract (LsAg). LsAg was
prepared as previously described (Gentil et al.,
2014).
2.4 Flow Cytometry, Fluorescence
Microscopy and Enzyme Linked
Immunosorbent Assay
After blocking with PBS containing 1% bovine
serum albumin and 0.1% rat IgG (Sigma-Aldrich, St.
Louis, USA) for 30 min, bmEos were washed and
stained with combinations of anti-SiglecF AL647
(BD Pharmingen, San Diego, USA), anti-
CD54/ICAM-1 AL488, and anti-CD193/CCR3 PE
(both BioLegend, San Diego, USA). Data were
acquired using a BD FACS Canto (BD Bioscience,
San Jose, USA) and analyzed by FlowJo v10
software (Tree Star, Ashland, USA).
For confocal fluorescence microscopy bmEos
were fixed with 3% formaldehyde fixative solution
for 20 min on 15 mm glass slides (P+W
Medizintechnik, Berlin, Germany) and stained with
rabbit anti- eosinophil cationic protein (ECP)
(Biorbyt Ltd, Cambridge, UK) for 1 h followed by 1
h staining with goat anti-rabbit FITC (Invitrogen,
Waltham, USA) and anti-SiglecF AL647. DAPI was
stained for 10 min (Sigma-Aldrich, Steinheim,
Germany). Z-stack pictures were taken with the
Zeiss LSM 710 and the ZEN 2.3 software (both Carl
Zeiss AG, Oberkochen, Germany).
Cytokine and chemokine concentrations were
determined from supernatants by ELISA according
to kit protocols (IL-6 and TNFα (eBioscience);
CXCL1 and CCL5 (R&D, Minneapolis, USA) using
a SpectraMAX 190 system and SoftMax Pro 6.5
software (Molecular Devices, Sunnyvale, USA).
2.5 Statistical Analysis
Statistical analysis was performed using Prism
GraphPad 5.01 (GraphPad Software, San Diego,
USA). Statistical significance was tested by Kruskal-
Wallis test followed by Dunn's Multiple Comparison
post hoc test. Significance is defined as p value <
0.05 and error bars represent means ± SEM.
3 RESULTS
3.1 Generation of Murine Bone-marrow
Derived Eosinophils
Flow cytometric analysis of in vitro generated
bmEos revealed a 98% purity of SiglecF
+
CCR3
+
cells (Fig. 1A, B). H&E staining as well as
fluorescence microscopy using anti-ECP, anti-
SiglecF and DAPI confirmed that bmEos had the
typical eosinophil appearance with eosin-stained
granule, U-shaped nucleus and contained ECP (Fig.
1C, D). The viability of bmEos was analyzed by
Annexin V and propidium iodide staining after
twelve days of culture and was consistently above
95% (Fig. 1E). In general, 50-80 million bmEos
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
6
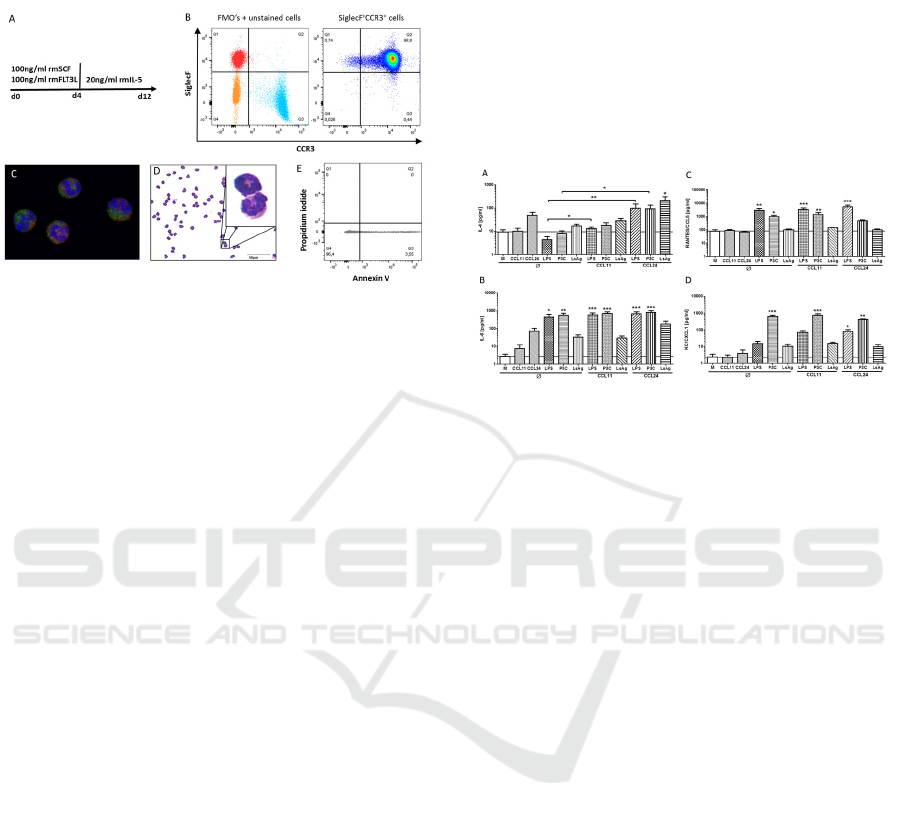
were obtained from one single donor mouse (data
not shown).
Figure 1: In vitro differentiation of bone-marrow derived
eosinophil granulocytes. Bone-marrow from tibiae and
femur of 6 week-old BALB/c mice were stimulated with
100ng/ml recombinant mouse SCF and recombinant
mouse FLT3L for four days followed by eight day
stimulation with recombinant IL-5 (A). Analysis of the
purity of SiglecF+CCR3+ eosinophils by flow cytometry
on day 12 (B). Fluorescence microscopy of eosinophils
stained with anti-SiglecF (red), anti-ECP (green) and
DAPI (blue) (C) and H&E staining of differentiated
eosinophils (D). Viability of differentiated eosinophils as
determined by Annexin V and propidium iodide staining
via flow cytometry (E).
3.2 CCL24 Modulates Cytokine and
Chemokine Release by Bone-marrow
Derived Eosinophils
Since eosinophils are predominantly recruited by the
chemokines CCL11 and CCL24, we investigated
their role on bmEos activation in vitro. BmEos were
stimulated for 24h with the filarial extract LsAg, the
TLR4 agonist LPS and the TLR1/2 agonist P3C, in
the presence or absence of CCL11 or CCL24 pre-
stimulation. IL-4 release by bmEos was not induced
by CCL11, LPS, P3C or LsAg stimulation alone, but
tended to be increased upon stimulation with
CCL24. Pre-stimulation of bmEos with CCL24
before LPS and P3C re-stimulation resulted in a
significantly increased release of IL-4 compared to
LPS- and P3C-only stimulated controls. Similarly,
CCL24 pre-stimulation significantly increased
LsAg-induced IL-4 release compared to
unstimulated controls (p<0.05). LPS and P3C
potently induced IL-6 and CCL5/RANTES by
bmEos (Fig. 2B, C). While pre-stimulation with
CCL11 had no impact on subsequent LsAg-, LPS- or
P3C-induced IL-6, CCL5 and CXCL1 release by
bmEos, CCL24 pre-stimulation reduced P3C-
stimulated CCL5 production and increased by trend
LsAg-induced IL-6 release (Fig. 2B, C). CXCL1
release was significantly induced by P3C but none
of the other stimulations alone (Fig. 2D). However,
pre-stimulation with CCL24 led to a significantly
increased CXCL1 release upon LPS re-stimulation
(Fig. 2D). Those results indicate that CCL24 pre-
stimulation affects bmEos responses to subsequent
stimuli.
Figure 2: CCL24 modulates cytokine and chemokine
release by bone-marrow derived eosinophils.
Concentrations of IL-4 (A), IL-6 (B), RANTES/CCL5 (C),
and KC/CXCL1 (D) in the supernatant after a total of 48h
stimulation. Cells were untreated or pre-stimulated with
CCL11 or CCL24 for 24 hours followed by re-stimulation
with LPS, Pam3CSK4 (P3C) or crude Litosomoides
sigmodontis adult worm extract (LsAg) for additional 24
hours. Data of two independent and pooled in vitro
experiments with 7 replicates are shown. Data is presented
as mean + SEM and analyzed for statistical significance
using Kruskal-Wallis test followed by Dunn’s posthoc test
(*p<0.05, **p<0.01, ***p<0.01).
3.3 CCL24 Pre-Stimulation Reduces
the Expression of CCR3
Since CCL24 pre-stimulation and P3C
stimulation induced bmEos activation, the
impact of CCL24 pre-stimulation on the
expression of CCR3 and ICAM-1 were
investigated. The expression of CCR3
significantly decreased upon CCL24 pre-
stimulation and was not altered by TLR2
stimulation (Fig. 3A, B). In contrast, ICAM-1
expression was increased by P3C stimulation,
but not altered by CCL24 pre-stimulation (Fig.
3C, D). These results indicate that bmEos react
upon TLR activation with an increased ICAM1
expression, which may facilitate their tissue
migration and reduce the expression of the CCR
for the major eosinophil recruiting factors after
pre-stimulation with CCL24.
Pre-stimulation of Bone-marrow Derived Eosinophils with CCL24 Alters Responses to TLR Ligands and Helminth Extracts
7
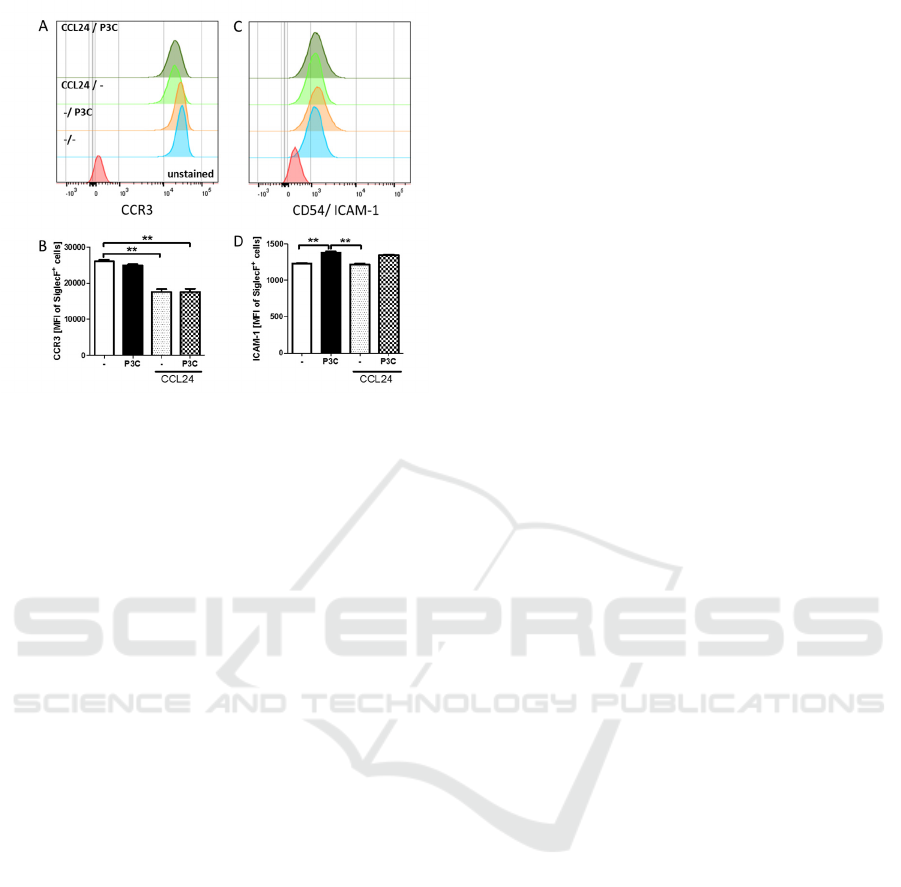
Figure 3: Treatment with CCL24 modulates CCR3
expression. Bone-marrow derived eosinophils were pre-
stimulated with CCL24 for 24h followed by six hours re-
stimulation with Pam3CSK4 (P3C). Histograms and MFI
of CCR3 (A, B) and CD54/ICAM-1 (C, D) SiglecF
+
eosinophils are shown. Data of two independent and
pooled in vitro experiments with 6 replicates are shown
and are presented as mean + SEM and analyzed for
statistical significance using Kruskal-Wallis followed by
Dunn’s post hoc test (*p<0.05, **p<0.01, ***p<0.01).
4 DISCUSSION AND
CONCLUSION
In this study we describe the in vitro generation
of bmEos and the impact of bmEos pre-
stimulation with CCL11/CCL24 on cytokine
and chemokine release in response to TLR
ligands and LsAg. The stimuli chosen for this
study induced different cytokine/chemokine
pattern from bmEos, with P3C and LPS
triggering CCL5 and IL-6 release, P3C
inducing CXCL1 production and CCL24 the
release of IL-4. Interestingly, LsAg-induced
cytokine/chemokine release by bmEos was only
present after pre-stimulation with CCL24,
resulting in increased IL-6 and IL-4 release.
CCL24 pre-stimulation also increased IL-4
responses after re-stimulation with P3C and
LPS. Such an effect by eotaxin to induce the
release of preformed IL-4 was also observed for
human eosinophils that was additionally
enhanced by IL-5 (Bandeira-Melo et al., 2001).
This indicates that in the context of increased
CCL24 concentrations, as they may occur
during type 2 inducing helminth infections,
eosinophils may be more prone to support type
2 immune responses independent on the
stimulus, which may render them more efficient
for protection against filarial infections (Gentil
et al., 2014). However, pre-stimulation with
CCL24 also triggered the release of pro-
inflammatory mediators like CXCL1 upon LPS
re-stimulation and IL-6 after LsAg re-
stimulation and bmEos responded to TLR2 and
TLR4 stimuli by the release of IL-6. Those
results suggest that bmEos may also support
anti-bacterial responses by triggering neutrophil
recruitment via CXCL1 and acute phase
responses, which can be in part enhanced by
CCL24 pre-stimulation. BmEos further reacted
upon TLR2 activation with an increased
ICAM1 expression, which may increase cell
contact with other leucocytes and promote
inflammation (Czech et al., 1993). In contrast,
CCR3 expression of BmEos was reduced by
CCL24 treatment independently of TLR2
stimulation suggesting two independent
mechanisms of eosinophil migration and
eosinophil activation (Humbles et al., 2002). In
summary, our data demonstrate that bmEos
possess characteristics that are known from ex
vivo isolated eosinophils and indicate that
CCL24 pre-treatment modulates eosinophil
responses.
SOURCES OF FUNDING
This work was funded by the German Research
Foundation (HU 2144/1-1). BB and AE are
supported by the Jürgen Manchot Stiftung,
Düsseldorf. AH is a member of the German
Center for Infection Research (DZIF) and of the
Excellence Cluster Immunosensation (DFG,
EXC 1023).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SKIC-MHS 2018 - The 2nd Syiah Kuala International Conference on Medicine and Health Sciences
8

REFERENCES
Bandeira-Melo, C., Sugiyama, K., Woods, L.J. and
Weller, P.F., 2001, Cutting edge: eotaxin elicits rapid
vesicular transport-mediated release of preformed IL4
from human eosinophils. Journal of immunology 166,
4813-7.
Chen, Z., Andreev, D., Oeser, K., Krljanac, B., Hueber,
A., Kleyer, A., Voehringer, D., Schett, G. and Bozec,
A., 2016, Th2 and eosinophil responses suppress
inflammatory arthritis. Nat Commun 7, 11596.
Czech, W., Krutmann, J., Budnik, A., Schopf, E. and
Kapp, A., 1993, Induction of intercellular adhesion
molecule 1 (ICAM-1) expression in normal human
eosinophils by inflammatory cytokines. The Journal of
investigative dermatology 100, 417-23.
Dyer, K.D., Moser, J.M., Czapiga, M., Siegel, S.J.,
Percopo, C.M. and Rosenberg, H.F., 2008,
Functionally competent eosinophils differentiated ex
vivo in high purity from normal mouse bone marrow. J
Immunol 181, 4004-9.
Fulkerson, P.C. and Rothenberg, M.E., 2013, Targeting
eosinophils in allergy, inflammation and beyond.
Nature reviews. Drug discovery 12, 117-29.
Gentil, K., Lentz, C.S., Rai, R., Muhsin, M., Kamath,
A.D., Mutluer, O., Specht, S., Hübner, M.P. and
Hoerauf, A., 2014, Eotaxin-1 is involved in parasite
clearance during chronic filarial infection. Parasite
Immunol 36, 60-77.
Humbles, A.A., Lu, B., Friend, D.S., Okinaga, S., Lora, J.,
Al-Garawi, A., Martin, T.R., Gerard, N.P. and Gerard,
C., 2002, The murine CCR3 receptor regulates both
the role of eosinophils and mast cells in allergen-
induced airway inflammation and
hyperresponsiveness. Proceedings of the National
Academy of Sciences of the United States of America
99, 1479-84.
Merino, C.A., Martinez, F.T., Cardemil, F. and Rodriguez,
J.R., 2012, Absolute eosinophils count as a marker of
mortality in patients with severe sepsis and septic
shock in an intensive care unit. Journal of critical care
27, 394-9.
Rosenberg, H.F., Dyer, K.D. and Foster, P.S., 2013,
Eosinophils: changing perspectives in health and
disease. Nature reviews. Immunology 13, 9-22.
Wu, D., Molofsky, A.B., Liang, H.E., Ricardo-Gonzalez,
R.R., Jouihan, H.A., Bando, J.K., Chawla, A. and
Locksley, R.M., 2011, Eosinophils sustain adipose
alternatively activated macrophages associated with
glucose homeostasis. Science 332, 243-7.
Pre-stimulation of Bone-marrow Derived Eosinophils with CCL24 Alters Responses to TLR Ligands and Helminth Extracts
9
