
The Potency of Binahong Leaves (Anredera cordifolia (Ten.) Steenis)
Subfraction with Ethanol 70% as an Antihyperuricemic Agent
Vera Ladeska, Ani Pahriyani and Monika Silviani Gunawijaya
Faculty of Pharmacy and Science, Universitas Muhammadiyah Prof. DR. HAMKA, Jakarta, Indonesia
Keywords: Uric acid, Anredera cordifolia (Ten.) Steenis, antihyperuricemic, Binahong leaves.
Abstract: Hyperuricemia is an abnormally high level of uric acid in a blood. Binahong leaves (Anredera cordifolia
(Ten.) Steenis) is one of the plants traditionally used as an antihyperuricemic remedy. This study aims to
determine the ethanol 70% subfraction activity of binahong leaves on the uric acid level of male white mice.
Antihyperuricemia assay was conducted for 36 days by dividing 24 mice into six groups. There are normal
control who was given standard feed and Na CMC 0.5%, positive control was given purine and allopurinol
0.8 mg/20 g BW and the assay group was given a purine feed and binahong leaves subfraction SF 3 with a
dose of 1.83 mg/20gBW, 3.60 mg/20gBW, and 5.40 mg/20gBW. Blood sampling was conducted by orbital
sinus after 2 hours from induction of potassium oxonate. Blood sampling was measured with an enzymatic
method using a clinical spectrophotometer. The result showed that the third dose had no significant
difference to the positive control with a percentage of decrease of 56.6%. The conclusion is that binahong
leaves subfraction has the same activity as an antihyperuricemic agent with allopurinol at dose 5.40
mg/20gBW.
1 INTRODUCTION
Indonesia is currently facing health problems of
Non-Communicable Diseases (NCDs), which tend
to increase every year. The Ministry of Health RI
(2016) reported that the number of death from NCDs
rose from 37% in 1990 to 57% in 2015. The
increased deaths can be caused by changes in diet
with imbalance nutrition (Kemenkes RI, 2011). One
sign of NCDs is due to dietary changes that lead to
increased levels of purine in the body causing
hyperuricemia (Purwaningsih, 2009). According to
an epidemiological survey conducted in Bandungan,
Central Java in a WHO-COPCORD collaboration
(2015) 4,683 samples aged between 15-45 years the
prevalence of hyperuricemia was 24.3% in males
and 11.7% in women. This disease can be grouped
into primary gout that commonly occurs (90% of
cases) and which cause is unknown clearly, and
secondary gout (10% of cases) that is experienced
by women after menopause due to hormone
imbalance (Daniati, 2015).
The number of side effects that arise from the
use of synthetic drugs and the long duration of
therapy has become a problem in the health field.
The development of herbal medicine can be a
solution to the problems considering the widespride
existance of medicinal plants in Indonesia. Binahong
leaves (Anredera cordifolia (Ten.) Steenis) are
traditionally used to treat gout, heart, diabetes,
stroke, asthma, acne, influenza, stiff, burn and so on
(Susetya, 2012). Binahong leaves contains active
compounds such as saponins, polyphenols,
flavonoids and polysaccharides (Rachmawati, 2008).
Flavonoid compounds are suspected to inhibit the
enzyme xanthine oxidase, which can inhibit the
formation of uric acid (Lin, 2002).
Binahong leaves extracted with ethanol 70% has
proven to decrease the level of uric acid at dose 200
mg/kgBW 91,83% from 3,56 mg/dl to 178 mg/dl
(Lidinilla, 2014). Fraction binahong leaves with
ethanol 70% at dose of 3,66 mg/20gBW has been
proven to decrease the level of uric acid from 4.048
mg/dl to 1.403 mg/dl. Based on the results of
previous research (Mutiarini, 2015), it is necessary
to further research into the subfraction stage in order
to produce a purer and cleaner compound of impure
compounds by column chromatography method.
The Potency of Binahong Leaves (Anredera cordifolia (Ten.) Steenis) Subfraction with Ethanol 70 .
DOI: 10.5220/0008240801430146
In Proceedings of the 1st Muhammadiyah International Conference on Health and Pharmaceutical Development (MICH-PhD 2018), pages 143-146
ISBN: 978-989-758-349-0
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
143

2 MATERIAL AND METHODS
2.1 Material
Mice, Vacuum Rotary Evaporator, Microcentrifuge,
column chromatography, Vortex Mixer, micropipet,
Clinical Spectrophotometer (Microlab 300), TLC
plate (silica gel GF 254), binahong leaves (Anredera
cordifolia (Ten.) Steenis), potassium oxonate (Sigma
Aldrich Chemical), allopurinol, ketamine.
2.2 Methods
2.2.1 Sample Preparation
Binahong leaves taken from BALITTRO was dried
by sun and covered with black cloth. Binahong
leaves were then powdered and sieved with mesh
number 40.
2.2.2 Mice Preparation
Twenty-four mice (Indonesian Institute of Sciences,
region Indonesia) were acclimatized and were fed
with standard feed. They were then divided into six
test groups of four mice for antihyperurecemic test.
2.2.3 Extraction
The simplicia powder was macerated with ethanol
70% for three times, then filtered. The resulting
mixture was collected and was evaporated with a
vacuum rotary evaporator until a viscous extract was
obtained.
2.2.4 Fractionation
A total of 170 g of binahong extract was fractionated
with n-hexane and ethanol - water in a separating
funnel – and was shaken for 15 minutes. After that it
was allowed to stand to form 2 layers (n-hexane at
the top and ethanol-water at the bottom). The
ethanol coating: water was fractionated back with an
ethyl acetate solvent, and then was rehydrated for 15
minutes. After that, it was allowed to stand to form
two layers (ethyl acetate at the top and ethanol:
water at the bottom). Each treatment was repeated
until the top layer was clear then all the fractions of
n-hexane, ethyl acetate and ethanol were evaporated
with a vacuum rotary evaporator.
2.2.5 Subfraction Proccess
An ethanol fraction was used as much as 20 g by
making wet column chromatography using a mixture
of n-hexane gradient solvent: ethyl acetate and ethyl
acetate - methanol in a ratio of 10: to 0:10.
2.2.6 Phytochemical Screening
Phytochemical screening was performed to test the
presence of groups of alkaloids, saponins, tannins,
flavonoids, and terpenoids with TLC method. The
stationary phase employed was a GF254 silica gel
plate with a mobile phase system and a detection
reagent adjusted to each of the detected compounds.
Silica Gel GF245 as a stationary phase and ethyl
acetate – methanol - ammonia (4-1-1) as a mobile
phase. The principle of separation on TLC based on
absorption and partition. TLC method was chosen
because can describe a chromatographic pattern of
samples, has a simple procedure and diverse motion
phases (Hanani, 2014).
2.2.7 Antihyperuricemic Test
From Day 15 to Day 28, all test group were induced
orally with high purine feeds of chicken liver juice
(200 g/100 ml) while the normal control was given
standard feed and 0.5 ml of Na-CMC. On Day 29
and Day 36, blood samples were taken. Uric acid
level was measured 2 hours after intraperitoneal
administration of potassium oxonate induction at 6
mg/20 g to all groups except Group I. From Day 29
to Day 36, the feed was continued to be given orally
according to the treatment group and was suspended
using Na-CMC. Serum was taken as much as 20 μl,
1000 μl of uric acid kit reagent (Human), then was
mixed in the vortex and was incubated for 5 minutes
at 37 ° C. The values of uric acid levels were read by
clinical spectrophotometer.
The following is the division of animal groups:
• Group I as a normal control (standard feed
with Na-CMC solution).
• Group II as a negative control (high purine
feed with Na-CMC solution).
• Group III as a positive control (high purine
feed with allopurinol at dose 0.8 mg/20gBW).
• Group IV as Dose 1 assay (high Purine feed
with binahong leaves subfraction at dose 1.83
mg/20gBW).
• Group V as a Dose 2 assay (high purine feed
with binahong leaves subfraction at dose of
3.60 mg/20gBW).
144

• Group VI as a Dose 3 assay (high purine feed
with binahong leaves subfraction at dose 5.40
mg/20gBW).
2.2.8 Statistical Analysis
Data was analyzed by one-way ANOVA which have
previously tested for normality and homogeneity.
The data then continued with Pos Hoc Tukey test to
know the differences between groups.
3 RESULTS AND DISCUSSION
One of the plants that can be used as herbal remedies
is the leaf of binahong (Anredera cordifolia (Ten.)
Steenis) which traditionally treats gout, heart
disease, diabetes, stroke, asthma, acne, influenza,
stiff, burn and so on (Susetya, 2012) . Binahong
contains active compounds such as saponins,
polyphenols, flavonoids and polysaccharides
(Rachmawati, 2008).
Table 1 shows the results of the extraction
process, The percentage of subfraction yields is
26.76%. The percentage of yields can show the
effectiveness in determining the appropriate method
for the process. The calculation of yield aims to find
out how much recovery of secondary metabolite
compounds in the subfraction. In the ethanol
subfraction results, 70% of the SF 3 binahong leaf
was carried out with a drying rate of 5.86 % in order
to see the quality of the obtained subfraction. The
compound may be a volatile oil residue, organic
solvent or water contained in there. The dry
shrinkage percentage of the binahong leaves was
less than 10 %. Thus, the result of drying drift is
<10%, it can be said the quality of the good
subfraction results.
The preparation of subfraction using column
chromatography resulted from 20 g ethanol fraction
obtained 5 stain which then continued checking of
flavonoid compound. There are four spot were
confirmed positive of containing flavonoids.
Flavonoids are phenolic compounds because their
color will change to purple light color if they are
added with bases or ammonia. In addition,
flavonoids contain conjugated aromatic compounds
because they exhibit strong absorption bands in UV
light (Harborne, 1987).
The results of flavonoid checking of the 5
subfraction stains. The positive results, which
contain flavonoids, are given a yellow circle (figure
1). The results of binahong leaves subfraction with
ethanol 70 % has the most flavonoid stain which
dominant among the other. The Rf values obtained
from SF 3 are 0,41; 0,87; and 0,94. Thus, SF 3 was
selected to continue testing of antihyperuricemia
activity because it has the dominant flavonoids
among other stains. Flavonoid compound are
suspected to inhibit xanthine oxidase which can
inhibit the formation of uric acid.
The next step is phytochemical screening. As
shown in Table 2, the binahong leaves contain
flavonoids compound only. Due to the process of
column chromatography with mixed motion phases
and variations in the phase comparison of motion.
The measurements of uric acid levels in white
Figure 1. Thin layer chromatography results with Rf
value (a) 0,94; (b) 0,87; (c) 0,90; (d) 0,43; (e) 0,41;
(f) 0,20.
Table 1. The results of sample preparation
No. Data Results
1. The wei
g
ht of sim
p
licia
p
owde
r
1,75 k
g
2. Thick extract of binahong
leaves
186,93 g
4. Fraction of binahon
g
leaves 46,21
g
5. Subfraction of binahong leaves
SF 3
5,35 g
6. Subrafraction of yield of
b
inahon
g
leaves SF 3
26,76 %
7. Subfraction of dry loss of
b
inahon
g
SF 3
5,86 %
Tabel 2. The result of phytochemical screening of
binahong leaves SF 3 subfraction yield using TLC
method.
No. Secondary Metabolite Result
1. Alkaloi
d
-
2. Flavonoi
d
+
3. Sa
p
onin -
4. Tanin -
5. Steroi
d
-
Note: (+) Positive, (-) Negative
The Potency of Binahong Leaves (Anredera cordifolia (Ten.) Steenis) Subfraction with Ethanol 70
145
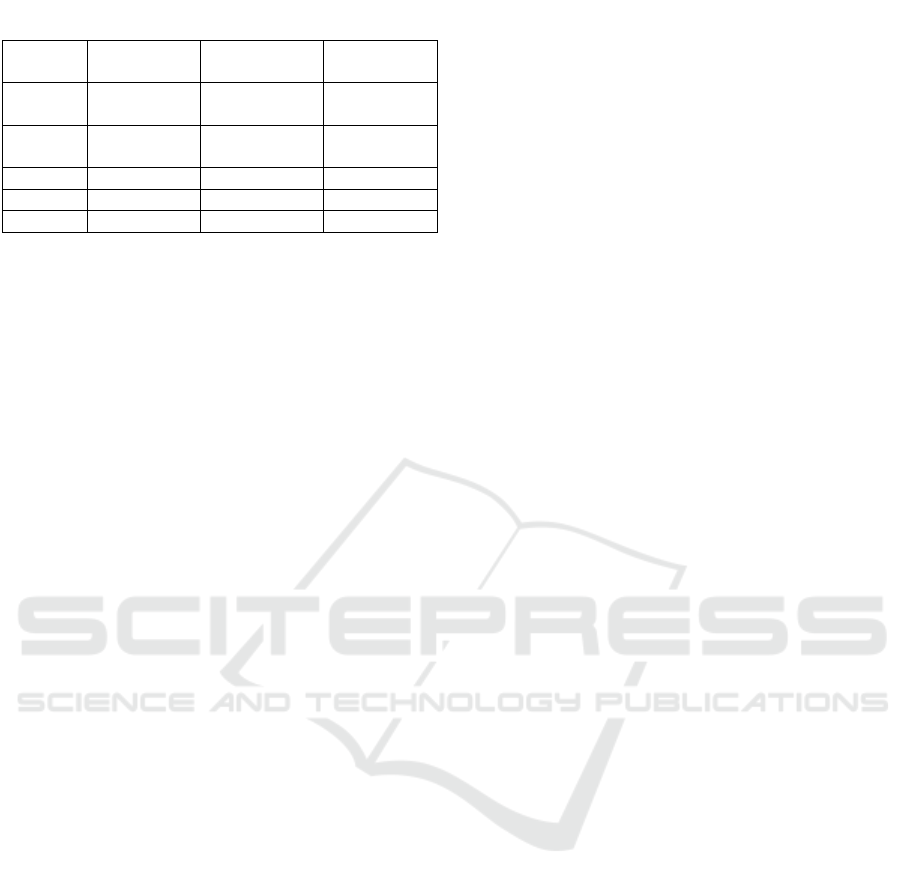
male mice induced high purine feed in the form of
chicken liver juice can be seen in Table 3 the
significance value of homogeneity test was 0.122
(p>0.05) which show that the data is homogeneously
distributed. The Shapiro-Wilk test indicates that the
data is normally distributed. The significant values
was less than 0.05 which stated a significant
difference in the treatment.
The result of the Tuckey test showed no
significant differences between negative control,
dose 1 dan dose 2 groups which is presumably
because the dose given is too small to affect the
small activity at this dose. There was also no
significant difference between positive control group
and dose 3 group. Binahong leaves subfraction with
dose 5.40 mg/20 gBW have equal lowering uric acid
activity with allopurinol group (table 3).
The binahong leaves subfraction has a flavonoid
compounds that have antihiperuricemic activity.
Allopurinol has the same mechanism of action with
flavonoids in reducing uric acid level. It contains
oxipurinol, the main metabolite, as xanthine oxidase
inhibitors that the conversion of hypoxanthine to
xanthine, and xanthine to uric acid (Sukandar et al.,
2009)
4 CONCLUSIONS
Based on the results of this research, ethanol
subfraction of 70% of binahong leaves (Anredera
cordifolia (Ten.) Steenis) SF 3 obtained has
antihyperurisemic activity. The activity of
decreasing uric acid level in dose 3 (5.40 mg/20
gBW) is proportional to allopurinol.
REFERENCES
Dianati NA.. 2015. Gout and Hyperuricemia. J Majority:
4(3).
Hanani E. 2014. Analisis Fitokimia. Jakarta: Buku
Kedokteran EGC.
Harbone BJ. 1987. Metode Fitokimia penuntun Cara
Modern Menganalisa Tumbuhan. edisi II. ITB Press.
Bandung.
Kementerian Kesehatan RI. 2011. Infodatin Pusat Data
Dan Informasi Kementerian Kesehatan
RI.http://www.depkes.go.id/ Diakses 9 Februari 2017.
Lidinilla NG. 2014. Uji Aktivitas Ekstrak Etanol 70%
Daun Binahong (Anredera cordifolia (Ten.) Steenis)
terhadap Penurunan Kadar Asam Urat dalam Darah
Tikus Jantan Yang Diinduksi dengan Kafeina. Skripsi.
Fakultas Kedokteran dan Ilmu Kesehatan UIN Syarif
Hidayatullah. Jakarta.
Lin CM, Chien SC,Yu CY, dan Jen KL. 2002. Moleculer
Modeling of Flavonoids that Inhibits Xanthine
Oxidase. Journal Biochemical and Biopshysical
Research Commnicartion.
Mutiarini A. 2015. Uji Aktivitas Fraksi Etanol 70% Daun
Binahong (Anredera cordifolia (Ten.) Steenis)
Terhadap Penurunan Kadar Asam Urat pada Mencit
Jantan Hiperurisemia. Skripsi. Fakultas Farmasi dan
Sains Universitas Muhammadiyah Prof. DR. Hamka.
Jakarta.
Purwaningsih T. 2009. Faktor-Faktor Risiko
Hiperurisemia (Studi Kasus Di RSU Kardinah Kota
Tegal). Tesis. Program Studi Magister Epidemiologi
Universitas Diponegoro. Semarang. Hlm.
Rachmawati S. 2008. Studi Makroskopik dan skrining
fitokimia daun Anredera cordifolia (Ten.) Steenis.
Tesis. Universitas Airlangga. Surabaya.
Sukandar EY, Retnosari A, Joseph IS, Adnyana IK,
Setiadi AAP, Kusnandar. 2009. Iso Farmakoterapi.
PT ISFI. Jakarta.
Susetya D. 2012. Khasiat dan manfaat daun ajaib
binahong. Pustaka Baru Press. Yogyakarta.
Tabel 3. The percentage of uric acid decline
Group Baseline ±
SD (mg/dl)*
Final Level ±
SD (mg/dl)*
Percentage
(%)
Normal
control
1,08 ± 0,06 1,07 ± 0.09 0,09
Positive
control
3,14 ± 0,27 1,18 ± 0,17 62,44
Dose 1 3,42 ± 0,55 2,48 ± 0,37 27,44
Dose 2 2,81 ± 0,35 2,03 ± 0,23 27,68
Dose 3 3,67 ± 0,23 1,59 ± 0,08 56,6
*Note: average of 5 experimental mice
146
