
The Effects of Liposomale Methylprednisolone
Palmitate on the Production of TNFα in Mice
Aprilita Rina Yanti Eff
1
, F. D. Suyatna
2
and Erni H. Purwaningsih
3
1
Department of Pharmacy, Faculty of Health Sciences, Universitas Esa Unggul, Jl,Arjuna Utara, West Jakarta, Indonesia
2
Department of Pharmacology and Therapeutics, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
3
Department of Pharmacy, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Keywords: Glucocorticoid, liposome, methylprednisolone palmitate, TNFα
Abstract: Introduction & Aim: Liposomes are used in this study as the carrier of an immunosuppressive drug, namely
methylprednisolone palmitate (MPLP), to reduce the drug side effects on various organ systems, such as
musculoskeletal, gastrointestinal, and cardiovascular. This study aimed to investigate the effects of low-dose
liposomal methylprednisolone palmitate (L-MPLP) on the in vitro and in vivo production of tumor necrosis
factor alpha (TNFα) derived from the culture of C3H mice spleen. Methods: For the in vitro culture, the
TNFα levels were identified using the splenic lymphocyte cultures. Meanwhile, for the in vivo culture, the
mice were divided into eight groups of five (5) mice randomly. Forty-eight hours after the drug
administration, these mice were sacrificed. The spleen was removed and used for lymphocyte culture. The
TNFα levels were measured with ELISA at a wavelength of 450 nm. Results: The in vitro and in vivo
assays showed that, when administered at the same dose, L-MPLP produced lower TNFα than
methylprednisolone (MPL). Conclusion: At small doses, L-MPLP can significantly inhibit the in vitro and
in vivo production of TNFα, as opposed to the control group MPL.
1 INTRODUCTION
Liposomes are phospholipid vesicles containing
polar and non-polar groups with a phospholipid
membrane structure similar to the cell membrane.
Liposomal vesicles are formed spontaneously when
lipids or phospholipids exposed to water. Liposomes
carry drug molecules in various ways, namely the
encapsulation of hydrophobic drug substances by
interacting with the lipophilic substance membrane
and the entrapment of hydrophilic substance inside
the vesicle (Jone, 2013).
As a drug carrier, liposomes have been tested in
animals and humans either by oral or parenteral
route. The oral administration of liposomes is
ineffective because they are enzymatically
hydrolyzed before entering the blood circulation.
They are also unstable in the intestines, and they can
interact with bile salts. Meanwhile, their parenteral
administration, especially intravenous, is more easily
monitored in terms of extravasation and uptake by
tissues. Several studies have used liposomes as a
drug carrier with the following reasons: liposomes
extend the half-life of drugs and increase the
distribution of drugs to the targeted organs, which
consequently reduce drug dose and minimize the
drug side effects (Anwekar et al., 2011; Ait-Oudhia
et al., 2014).
MPL is a lipophilic glucocorticoid containing a
hydroxyl (-OH) group at C
21
which makes it
amphoteric and able to form micelles. The formation
of micelles makes MPL easily detached from the
liposome membrane, resulting in unstable
liposomes. Benameur et al. (1993) incorporate
dexamethasone palmitate (DMP) into liposomes by
replacing the -OH group at C
21
with a palmitate
group, forming a more lipophilic drug and creating a
better DMP interaction with liposome membrane
than dexamethasone alone. Shaw et al. (1976) also
manage to incorporate cortisone in the form of
cortisone palmitate into liposomes. Following the
success of Benaumer et al. (1993) and Shaw et al.
(1976) and relying on the similarity of the basic
structures of dexamethasone- and cortisone-
incorporated methylprednisolone, this research
hypothesized that the addition of a palmitate group
to the C
21
of methylprednisolone would give similar
results. The initial experiment in our research proved
Eff, A., Suyatna, F. and Purwaningsih, E.
The Effects of Liposomale Methylprednisolone Palmitate on the Production of TNFÎ
´
s in Mice.
DOI: 10.5220/0008239700770083
In Proceedings of the 1st Muhammadiyah International Conference on Health and Pharmaceutical Development (MICH-PhD 2018), pages 77-83
ISBN: 978-989-758-349-0
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
77

that the preparation of liposomal methylprednisolone
palmitate (L-MPLP) using phosphatidylcholine from
egg yolk (Egg-Yolk Phosphatidyl Choline/EPC)
resulted in an incorporation of about 70% and that
the combination of EPC and 2.5 mol% tetraether
lipid (TEL) increased the incorporation of MPLP
into the liposome membrane to 95%.
In spite of having strong anti-inflammatory
effects, dexamethasone has a long half-life,
stimulates activity in bone demineralization, and
suppresses the growth factor better than
methylprednisolone; therefore, it is not suitable for
long-term therapy, for example in post-organ
transplantation (Becker, 2013). This condition is the
reason behind the exclusion of dexamethasone in our
study. Immunosuppressants play an important role in
preventing the recipient’s body from rejecting
transplanted organ or tissue and in treating several
autoimmune diseases. Immunosuppressive drugs
promote the success of organ transplants, such as
kidney, bone marrow, liver, heart, pancreas, and
lungs. Some autoimmune diseases and disorders,
such as hemolytic anemia, idiopathic
thrombocytopenia purpura, Hashimoto's thyroiditis,
systemic lupus erythematosus, acute
glomerulonephritis, and acquired hemophilia, have
improved with the use of this drug (Luisa and
Piedras, 2013).
Liposomes are used as drug carriers, which in
this case is methylprednisolone palmitate (MPLP),
to reduce the drug side effects and retain the
therapeutic function of the resultant liposomal drug
even when administered at a small dose. MPLP is a
novel compound created using the same synthesis
method as that of dexamethasone palmitate
developed by Benameur et al. (1993), and it has
been successfully incorporated into the liposome
membrane by Purwaningsih et al. (2007), forming
methylprednisolone palmitate (L-MPLP). However,
this new compound has never been evaluated for its
immunosuppressive effect. The initial step of the
biological activity test of L-MPLP is assessing its
immunosuppressive effects on lymphocyte
proliferation. This new compound is expected to
inhibit the proliferation of lymphocytes, which is
indicated by the decreased production of TNFα, as
the mitogen ‘concanavalin A’ stimulates the other
glucocorticoids in cultured lymphocytes.
TNFα is a cytokine playing a major role in the
activation of immune reactions, including the
specific and non-specific immune system. The other
roles of TNFα are to stimulate endothelial cells,
express adhesion molecules, activate inflammatory
cells, and stimulate other cells in expressing major
histocompatibility (MHC) class I molecules on the
cytotoxic T cells (Keystone and Ware, 2010).
Lymphocyte proliferation test mainly aims to
determine the proliferation ability of lymphocyte
cells after mitogen stimulation with or without
drugs. Some mitogens stimulate a specific
subpopulation of lymphocytes. For instance,
concanavalin A (con-A) stimulates T lymphocyte
cells (Abbas et al., 2014).
This study aimed to evaluate the biological
effects of L-MPLP by measuring the TNFα levels
in the lymphocyte cultures of C3H mice spleen after
the administration of L-MPLP with different
concentrations, namely 0.005 mM, 0.05 mM, and
0.5 mM, and 48 hours after the intravenous
administration of L-MPLP at different doses, i.e., 2,
8, and 16 mg/kg BW.
2 MATERIALS AND METHODS
2.1 Materials
The liposomes and liposomal methylprednisolone
palmitate (L-MPLP) were made fresh from Egg-
Yolk Phosphatidylcholine (EPC), the Tetraether
Lipid (TEL) was obtained from Purwaningsih et al.
(2007), and the MPLP was donated by Bernina
Biosystems GmBH. The methylprednisolone-Na
succinate (Solu-Medrol) was purchased from
Upjohn. This research also used ethyl acetate,
methanol, chloroform PA, NaOH, HCl, Tris buffer
solution (pH 7.4) from Merck (filtered through a
Millipore membrane before use), and Aquabidest
(sterilized water) from IKA Farma. The other
materials were RPMI 1640 (pH 7.2-7.4, Gibco), fetal
bovine serum/FBS (ICN Flow), gentamicin (Gibco),
fungizone (Gibco), sodium bicarbonate (ICN Flow),
concanavalin A (Sigma) as a mitogen, nylon wool
(Biotest), nylon mesh, 2M HCl, aqua Millipore,
aquabidest, 70% alcohol, sterile cottons, candles,
CO
2
gas from Perum Aneka Gas, and TNFα ELISA
kits (Quantikine). The male C3H mice (12-16 weeks
old, 20-22 gr in weight) were obtained from the
Department of Anatomic Pathology, Faculty of
Medicine, Universitas Indonesia. Before the
experiment, the test animals were acclimatized in
captivity in the Laboratory of Pharmacology,
Faculty of Medicine. They were given ad libitum
access to food and drink. The study approval (No
556/PT02.FK/ETIK/2012) was obtained from the
Ethics Committee, Faculty of Medicine, Universitas
Indonesia before the study began.
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
78

2.2 Methods
2.2.1 In Vitro Measurement of TNFα Levels
The TNFα assay was performed in the splenic
lymphocyte cultures using the Tris buffer solution
and liposomes as controls. RPMI solution containing
1x10
-6
cells/ml and 2.5 g/ml concanavalin A were
added to each of the following groups: the liposome
solution (20 ml, 0.5 mM), MPL, and the three L-
MPLP concentrations (0.5 mM, 0.05 mM, and
0.005 mM). After 48-hour incubation, the cultures
were centrifuged at 2,000g for 10 min. The
supernatants were discharged and stored at -20
0
C
before being used for the measurement of TNFα
levels using ELISA at a wavelength of 450 nm.
2.2.2 In Vivo Measurement of TNFα Levels
Drug Administration
The drug was administered intravenously to each
mouse based on the group division, i.e., eight (8)
groups of 5 mice, via vena lateralis in the tail. The
group division was as follows: Group I was used as a
control (Tris buffer, 5ml/kg); Groups II, III, and IV
were administered with methylprednisolone sodium
succinate/MPL at different concentrations, i.e., 8, 16,
and 32 mg/kg BW, respectively; Groups V, VI, and-
VII were given liposomal methylprednisolone (L-
MPLP) at different concentrations, i.e., 2, 8, and 16
mg/kg BW, respectively; and Group VIII was treated
with 0.5 mM liposomes. Forty-eight hours after drug
administration, these mice were sacrificed by
exposing them to the aether. Their spleens were
removed using scissors, and the lymphocyte culture
was collected with tweezers.
The Lymphocyte Culture
The spleens were placed in a sterile 60mm Petri dish
containing 5 ml of RPMI medium. To get the
lymphocyte cell suspension, the cell was filtered
through a sterile nylon mesh, and the erythrocytes
were lysed with ammonium chloride buffer solution.
The amount of T lymphocytes was enriched by
flowing the cell suspension through a nylon wool
column, which had been soaked in 2M HCl
overnight and then washed with distilled water, for
5-6 times and aerating it to allow drying.
Nylon wool fibers were separated with tweezers
and inserted into a polyethylene plastic syringe. As
much as 0.3 gram of nylon wool was inserted into a
5ml syringe to form the column for the spleen cell
suspension. The insertion was conducted with
moderate pressure and followed by the slow release
of the piston. The column, piston, and needle syringe
were sterilized in an autoclave for 15 minutes at a
temperature of 110-115
0
C. The column was
preincubated with RPMI 1640 medium containing
5% fetal bovine serum (FBS). The cell suspension
was resuspended to achieve a population of 5x10
6
cells/ml. It was loaded onto the column and, then,
incubated at 37
0
C for 60 minutes. The unretained
lymphocytes were eluted with a medium (eluent) as
much as one volume of the syringe. The eluate (i.e.,
T lymphocytes) was washed two (2) times and
centrifuged at 2,000g for 5 minutes. Afterward, a
population of 1x10
6
cells/ml was prepared from the
cell suspension. Lymphocytes with a concentration
of 1x10
6
cells/ml were maintained in RPMI 1640
medium containing 5% FBS, fungizone, gentamicin,
and concanavalin A (10 µg/ml). The in vitro cultures
were created by adding MPL and L-MPLP with four
different concentrations (i.e., 0.005 mM, 0.05 mM,
0.5 mM, and 0.5 mM) to the mice spleen. After a 48-
hour incubation, the cultures were centrifuged at
2,000g for 10 min. The supernatants were
discharged and stored at -20
0
C before being used for
the measurement of TNFα levels using ELISA at a
wavelength of 450 nm (Wohler & Barnum, 2010;
Bhattacharjee & Das, 2008)
2.2.3 Data Analysis
The data obtained from in vitro and in vivo cultures
were analyzed in SPSS 20.0. The mean values of the
measurement results of the groups were compared
using One-way ANOVA and expressed as
mean±SD. The p-value of <0.05 meant that the
difference between the mean values was statistically
significant.
3 RESULTS AND DISCUSSION
The results of the TNFα level measurement from the
in-vitro culture are shown in Figure 1A, while the
percentage of TNFα level in each group to the
TNFα level in the control group (represented as %
levels of TNFα) is depicted in Figure 1B. The TNFα
levels and the % levels of TNFα from the in-vivo
culture can be seen in Figure 2A and Figure 2B,
respectively.
The statistical test results showed that percentages of
the TNFα levels in Groups MPL1, MPL2, MPL3,
L-MPLP1, and liposomes were not significantly
different from the control group (p>0.05).
Meanwhile, the percentages of the TNFα levels in
Groups L-MPLP2 and L-MPLP3 were significantly
The Effects of Liposomale Methylprednisolone Palmitate on the Production of TNFÎ
´
s in Mice
79
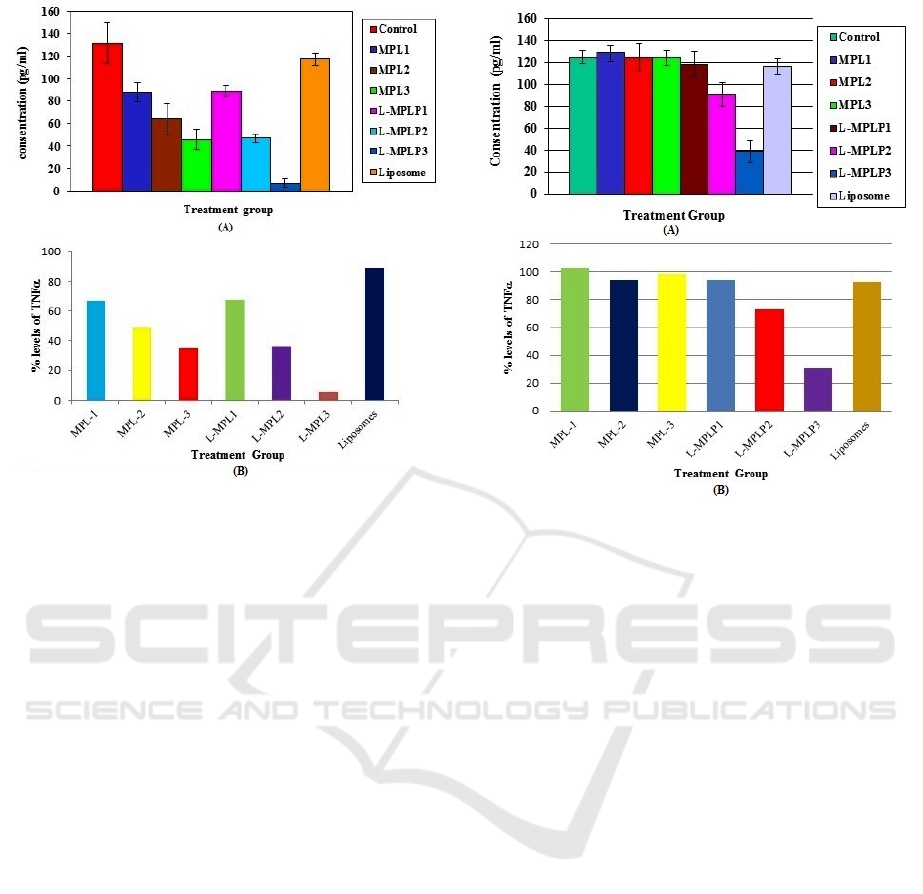
different (p <0.05) from the control group (without
the administration of drugs or MPL). The
administration of L-MPLP at a dose of 2 mg/kg BW
resulted in an equal TNFα level to MPL given at a
dose of 16 mg/kg BW, i.e., 94.85% (see Figure 2B).
Meanwhile, at the same dose of 8 mg/kg BW, the
treatment using L-MPLP produced lower TNFα than
MPL by 1.5 times. At a dose of 16 mg/kg BW, L-
MPLP yielded TNFα level 3 times lower than MPL
at the same dose.
Several theories suggest that liposomes as drug
carriers can extend the half-life of drugs and increase
the distribution of drugs into the organ selectively so
that the drug dose can be minimized (Sercombe et
al., 2015; Mishina et al., 1994; Binder et al., 1994).
Mishina et al. (1994) state that, when compared to
methylprednisolone (MPL) at a dose of 2 mg/kg
BW IV), the same dose of liposomal
methylprednisolone (L-MPL) in male Sprague-
Dawley rats can prolong the half-life from 0.48
hours to 30.13 hours and increase the distribution
volume from 2.1 L/kg to 21.87 L/kg. However, the
use of L-MPLP at a dose of 2 mg/kg BW in our
study did not exhibit any biological effects on mice.
This finding contradicts the research conducted by
Mishina et al. (1994) and Binder et al. (1994) where
the administration of L-MPL at this dose exhibits
biological effects. These two studies successfully
examine the immunosuppressive effects of the same
dose of L-MPL in male Sprague-Dawley rats, as
evident in the increase of survival to 30 days after a
heart transplant (instead of only 10-day survival in
the control group).
Although Mishina et al. (1994) and Binder et al.
(1994) identify the immunosuppressive effects of
MPL without measuring the TNFα level, these two
studies provide a conclusion that at a dose of 2
mg/kg BW, L-MPL gives a good
immunosuppressive effect. Meanwhile, in our study,
the use of L-MPLP at the same dose has no
biological effects on male C3H mice because of
several reasons. The first is the different strains of
Figure 1: (A) TNFα levels (mean ± SD) and (B) the
percentages of TNFα levels in each group compared to
the TNFα level in the control group (in vitro culture).
(Control group) Tris Buffer 5 ml/kg BW; (MPL1) 0.5 mM
methylprednisolone sodium succinate; (MPL2) 0.05 mM
methylprednisolone sodium succinate; (MPL3) 0.005 mM
methylprednisolone sodium succinate; (L-MPLP1) 0.5
mM liposomes methylprednisolone palmitate; (L-MPLP2)
0.05 mM liposome methylprednisolone palmitate; (L-
MPLP3) 0.005 mM liposome methylprednisolone
palmitate
Figure 2: (A) TNFα Level (mean ± SD) and (B) the
percentages of TNFα levels in each group compared to
the TNFα level in the control group (in vivo culture).
(Control group) Tris Buffer 5 ml/kg BW; (MPL1) 0.5 mM
methylprednisolone sodium succinate; (MPL2) 0.05 mM
methylprednisolone sodium succinate; (MPL3) 0.005 mM
methylprednisolone sodium succinate; (L-MPLP1) 0.5
mM liposomes methylprednisolone palmitate; (L-MPLP2)
0.05 mM liposome methylprednisolone palmitate; (L-
MPLP3) 0.005 mM liposome methylprednisolone
palmitate
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
80

the test animals. This study uses mice with C3H
strain, whereas Mishina et al. (1994) and Binder et
al. (1994) use the Sprague-Dawley strain. Such
difference in the test animal species leads to
dissimilar sensitivity to glucocorticoids. The second
reason is the type of the liposome used in the
experiments. These two studies use liposome made
from a combination of EPC and
phosphatidylglycerol. Meanwhile, the liposome in
our research is small-sized with a diameter of 73 nm,
and it is made from a combination of EPC and TEL.
The different types of phospholipids determine the
size and diameter of the liposome, which, in turn,
affect the speed of the drug uptake (Sercombe et al.,
2015; Shashi et al., 2012).
The small unilamellar liposome vesicles
(SUVs) are absorbed at a slower pace compared to
the large-sized ones (LUVs). Therefore, the
circulation time of liposomal SUVs is longer than
the liposomal LUVs. Liposomes made from the
combination of EPC and phosphatidylglycerol are
medium-sized (MUVs), i.e., about 100 nm
(Sercombe et al., 2015; Kumar et al., 2012), whereas
the liposomes in our research are small-sized
(SUVs), i.e., about 73 nm (Purwaningsih et al.,
2007). The third reason is that both Mishina et al.
(1994) and Binder et al. (1994 use MPL, which is
already widely used and known for its effects,
whereas our study chooses a novel compound
(MPLP) that is expected to be a pro-drug. Therefore,
even though administered during the same period,
MPLP has not shown its effect yet, as opposed to
MPL.
Methylprednisolone (MPL) sodium succinate is
a pro-drug (an MPL derivative) that is rapidly
hydrolyzed to methylprednisolone with a half-life of
2.5 hours in humans when administered at a dose of
1 mg/kg BW. At higher doses than 10 mg/kg BW,
the half-life becomes longer, i.e., up to 3.6 hours.
The half-life of MPL sodium succinate after the
intravenous administration to the C3H mice was 10-
30 minutes. MPL sodium succinate did not decrease
the TNFα levels due to its short half-life.
Accordingly, after 48 hours of administration, the
drug levels in the blood were very low. This finding
is similar to several references that mention that the
administration of a single dose of glucocorticoids
reduces lymphocytes, monocytes, basophils, and
eosinophils for 4-6 hours and returns them to their
normal levels after 48 hours.
Figure 2A shows that L-MPLP is still exhibiting
an effect on T lymphocytes and reducing the TNFα
levels after 48 hours of treatment. This condition
shows that the use of liposomes as drug carriers can
extend the half-life of MPLP, but the length of the
half-life of MPLP remains unclear. Using different
concentrations of dexamethasone (DEX), namely at
a concentration of 0.3, 0.8, and 10 μg/ml, against the
mitogen-induced proliferation of lymphocytes in
rats, Miller et al. (1991) explain that DEX influences
T cell proliferation and the in vitro mitogen-induced
proliferation of lymphocyte in cell cultures.
As seen in Figure 1A, the different
concentrations of MPL and L-MPLP (i.e., 0.5, 0.05,
and 0.005 mM) added to the cultures before being
incubated for 48 hours inhibit lymphocyte
proliferation, which is significantly different from
the control group (without drug administration,
p<0.05). The figure also shows that at a
concentration of 0.5 mM, L-MPLP inhibits the
lymphocyte proliferation by decreasing the TNFμ
levels, which are significantly different from the
control MPL at the same concentration (p<0.05).
Furthermore, the application of liposome without
any immunosuppressive drugs at a concentration of
0.5 mM does not decrease the TNFα levels (p>0.05).
This finding shows that liposomes do not affect
lymphocyte proliferation and TNFα level. It also
indicates that 0.5 mM liposomes comprised of a
combination of EPC and TEL are not toxic to C3H
mice. From the results of a toxicity study of 6 μg/ml
TEL in L5178Y murine lymphoma cells (EMAT
cells) and mutagenicity or antimutagenicity tests of
Salmonella typhimurium strain TA 100, Freisleben
et al. (1993) affirm that TEL is nontoxic and
nonmutagenic.
The additions of MPL and L-MPLP to the
cultures before 48 hours of incubation resulted in the
proliferation of lymphocyte that reduced the TNFα
levels significantly, as opposed to the controls (p
<0.05). This condition is explainable by the ability
of glucocorticoids to suppress lymphocyte
proliferation. According to Cidlowski (2013), the
sensitivity of glucocorticoid receptor varies
depending on the antigen or mitogen used.
Benameur et al. (1995) state that liposomes as
drug carriers are useful for reducing the
administration dose of dexamethasone palmitate
while retaining its therapeutic effects. As an initial
test to assess the biological activity of liposomal-
dexamethasone (DMP-SUVs), they employ
lymphocyte proliferation test and interferon gamma
level (IFNγ) measurement and compare the results to
the use of dexamethasone (DEX) without liposomes
at 1/6 times of the dose of DEX. Based on the
proliferation test and measurement results, they
conclude that DMP-SUVs inhibit lymphocyte
proliferation and reduce the levels of IFNγ six (6)
The Effects of Liposomale Methylprednisolone Palmitate on the Production of TNFÎ
´
s in Mice
81

times greater than dexamethasone alone. In other
words, the use of DMP-SUVs in therapy can be
reduced without altering their pharmacological
effects because liposomes carry the drug to the
targeted organs, especially the ones that are rich in
reticulum-endothelial systems like liver and spleen
(Anwekar et al., 2011). The sustained drug release
offered by liposomes prolongs the drug exposure in
the cells, allowing more drug cellular uptake (Jone,
2013).
In our study, the administration of L-MPLP at a
dose of 2 mg/kg to the C3H mice inhibited the
formation of TNFα. This result was equal to the
administration of MPL at a dose of 16 mg/kg.
Meanwhile, the administrations of L-MPLP at the
doses of 8 mg/kg and 16 mg/kg inhibited the
formation of TNFα, respectively, by 1.4 times and 3
times greater than MPL at the same doses.
The in vitro administrations of 0.005 mM L-
MPLP and MPL to the cell cultures inhibited the
formation of TNFα by nearly equal numbers.
Meanwhile, the applications of 0.05 mM and 0.5
mM L-MPLP inhibited the formation of TNFα,
respectively, by 1.4 and 6.6 times greater than MPL
at the same concentrations.
Tumor Necrosis Factor (TNF) plays an important
role in a broad range of immune and inflammatory
processes, including cellular activation, survival, and
proliferation, as well as cell death by necrosis and
apoptosis (Keystone, 2010). Glucocorticoids are
used as immunosuppressant drugs that inhibit or
prevent cellular and humoral immunity. They
suppress cellular immune responses that suppress
hinder T cell proliferation by inhibiting enzymes
with the following cytokines: IL-1, IL-2, IL-3, IL-4,
IL-5, IL-6, IL-8, and TNF. Glucocorticoids suppress
humoral immunity’s response and lead to the
expression of IL-2 and IL-2 receptors on B cells
(Rathee et al., 2012). Applying 10 µg/ml
lipopolysaccharide to male CBA/J mice in an in vivo
study, Nguyen et al. (1990) affirm that the 2-week
administration of cyclosporine at a dose of 75 mg/kg
decreases the levels of TNFα, which are
significantly different from the TNFα level in the
control group. They also affirm the inhibition of in
vitro TNFα formation by using murine macrophages
that are stimulated with lipopolysaccharide and
administered with cyclosporine at a dose of 0.001 to
1 g/ml. This inhibition is significantly different from
the one exhibited in the control groups (without
cyclosporine) (Nguyen et al., 1990). Our research
used a small dose of liposomal methylprednisolone
palmitate, which also inhibited the formation of
TNFα. The resulting TNFα level was significantly
different from the one in the control groups. In this
case, such administration is expected to reduce the
side effects and toxicity of methylprednisolone
palmitate.
4 CONCLUSIONS
A small dose of liposomal methylprednisolone
palmitate (L-MPLP) can significantly inhibit both in
vivo and in vitro productions of TNFα, as opposed
to the control group, i.e., methylprednisolone
(MPL).
ACKNOWLEDGMENTS
The authors would like to express their gratitude to
Prof. Dr. Ernie H. Purwaningsih and Prof. F.D.
Suyatna, Ph.D. from the Faculty of Medicine,
Universitas Indonesia, for their invaluable helps in
providing liposomes and liposomal
methylprednisolone palmitate (L-MPLP) for this
study.
REFERENCES
Abbas, A.K, Lichtman, A.H., Pober, J.S. (eds)., 2014.
Cytokines. In: Cellular and Molecular Immunology.
8th ed, WB Saunders, pp. 226-242.
Ait-Oudhia, S., Mager, D.E., & Straubinger, R.M., 2014.
Application of pharmacokinetic and pharmacodynamic
analysis to the development of liposomal formulations
for oncology. Pharmaceutics,6(1), pp.137–174.
Anwekar, H., Patel, S. & Sinhai, A.K., 2011. Liposome- as
drug carriers. International Journal of Pharma Life
Sciences, 2(7), pp. 945–951.
Becker, D.E., 2013. Basic and clinical pharmacology of
glucocorticosteroids. Anesthesia Program, 60(1), pp.
25-31.
Benameur, H., De Gand, G., Brasseur, R., Van Vooren,
J.P., Legros, FJ., 1993. Liposomes incorporated
dexamethasone palmitate. Chemical and physical
properties. International Journal of Pharmacy, 89, pp.
157- 167.
Bhattacharjee, K. and Das, S.K., 2008. Morphology of
immunocompetent cells isolated from spleen of Bufo
himalayanus (Günther). Indian Journal Experiment
Biology, 46 (March), pp.191–195.
Binder, J., Mishina, E.V., Jusko, W.J, Kupiec-Weglinski,
J.W., 1994. Prolongation of cardiac allograft survival
in rats by liposomes-encapsulated methylprednisolone.
Transplantation, 58(5), pp. 633-635.
Cidlowski, A.J, A.H.R., 2013. The Biology of the
Glucocorticoid Receptor: New Signaling Mechanism
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
82

in Health and Disease. Journal of Allergy and Clinical
Immunology, 132(5), pp.1033–1044.
Freisleben, H.J, Neisser, C., Hartmann, M., Rudolph, P.,
Geck, P., Ring, K., Muller, W.E.G., 1993. Influence of
the main phospholipid (MPL) from Thermoplasma
acidophilum and liposomes from MPL on living cells:
cytotoxicity and mutagenicity. Journal of Liposome
Research, 3, pp.817-833.
Jone, A., 2013. Liposomes : A short Review, 5(9), pp.181–
183.
Keystone, E.C. & Ware, C.F., 2010. Tumor Necrosis
Factor and Anti-Tumor Necrosis Factor Therapies.
Journal Rheumatology, 85(0), pp.27–39.
Kumar, D.P., et al., 2012. Liposomes: An Overview.
Journal Pharmaceutical Sciences Innovation, 1(June),
pp. 27–34.
Luisa, A. & Piedras, R., 2013. Clinical Pharmacology and
Therapeutic Drug Monitoring of Immunosuppressive
Agents. Current Issues Future Kidney Transplantation.
Miller, H.A., Spencer, L.R., Trestman, L.R, Kim Christin.,
1991. Adrenal steroid receptor activation in vivo and
immune function. American Physiology Society, (E1),
pp.26-31.
Mishina, E.V., Binder, J., Kupiec-Weglinski, J.W., 1994.
Effect of liposomal methylprednisolone on heart
allograft survival and immune function in rats. Journal
Pharmacology Experimental and Therapy, 271(2), pp.
868-874
Nguyen, T.D, Eskandari, K.M., Deforge, E.L, Raiford,
L.C., 1990. Cyclosporin a modulation of tumor
necrosis factor gene expression and effects in vitro and
in vivo. Journal of Immunology, 144, pp.3822-3828.
Purwaningsih, E.H., Arozal, W., Jusman, S.W.A., 2007.
Uji stabilitas fisik, kimia dan biologi terhadap
formulasi terbaru liposom tetra eter lipid (EPC-TEL
2.5) sebagai pembawa obat (drug carrier). Makara
Kesehatan, 11 (2), pp. 84-89
Rathee, P., Chaudhary, H., Rathee.,S, Rathee., D and
Kumar, V., 2013. Immunosuppressants: A Review.
Pharma Innovation. January, 1(12), pp.90-101
Sercombe, L. Veerati, T., Moheimani, F., Wu, S.Y., Sood,
A.K., Hua, S., 2015. Advances and challenges of
liposome assisted drug delivery. Frontier
Pharmacology, 6(Dec), pp.1–13.
Shashi, K., Satinder, K., Barat, P., 2012. Review Article
A Complete Review On Liposomes. International
Research Journal of Pharmacy, 3(7), pp.10–16.
Shaw, I.H., Knight, C.G., Dingle, J.T., 1976. Liposomal
retention of modified anti-inflammatory steroid.
Journal Biochemistry, 58, pp. 473- 4766.
Wohler, J.E., & Barnum, R.S., 2010. Nylon Wool
Purification Alters the Activation of T Cells.
Molecular Immunology, 46(5), pp.1007–1010.
The Effects of Liposomale Methylprednisolone Palmitate on the Production of TNFÎ
´
s in Mice
83
