
Hepatoprotective Effect of Mountain Papaya (Vasconcellea
pubescens A.DC.) Fruit Extract against Acetaminophen-Induced
Acute Liver Damage
Heru Sasongko
*1,2
, Diah Pratiwi
1
, Trias Amartiwi
1
, Nur Rohman Efendi
1
and Sugiyarto
2
1
Department of Pharmacy Faculty of Mathematics and Natural Sciences Universitas Sebelas Maret. Jl. Ir. Sutami 36A
Surakarta 57126, Central Java Indonesia
2
Department of Biology Faculty of Mathematics and Natural Sciences Universitas Sebelas Maret. Jl. Ir. Sutami 36A
Surakarta 57126, Central Java Indonesia
Keywords: acetaminophen, hepatoprotective, mountain papaya, flavonoid, Vasconcellea pubescens
Abstract: The liver is a main organ of drugs metabolism in humans. Many studies have reported that acetaminophen
has an effect on liver injury. Targeting mitochondrial oxidant stress is a promising therapeutic option for
acetaminophen hepatotoxicity. Previous study has shown that mountain papaya (Vasconcellea pubescens
A.DC.) fruit has an effect against lipid peroxidation activity. This research aims to know the
hepatoprotective effect of mountain papaya fruit ethanolic extract (MPFE) in rats after induced by
acetaminophen. The rats were divided into six groups, with group I not administered with any treatment,
group II administered with suspension of 0.25% CMC-Na as negative control, group III was administered
with suspension of silymarin as positive control. Groups IV, V, and VI were given MPFE with variation
doses 120; 240 and 480 mg/kg body weight for 14 days. At day 14th, all groups except the normal group
were induced with acetaminophen in toxic dose (2 g/kg body weight). The liver injury was measured by
ALT, AST, bilirubin, ALP value and liver histology profile. The results showed that MPFE could
significantly decrease liver cell injury (p <0.05) by ALT, AST and liver histology profile parameters in
which each dose has the same ability.
1 INTRODUCTION
The liver is the largest gland of the carbohydrates,
lipids, proteins and xenobiotic and drug
detoxification (Kumar et al., 2013). As the center of
metabolism in the body, liver is vulnerable to
chemicals exposure which makes this organ
susceptible to injury (Muriel, 2017). Acetaminophen
overdose is the most frequent cause of liver injury
and acute liver failure in many countries (Jaeschke
and Ramachandran, 2018; Larsen and Wendon,
2014). The formation of a reactive metabolite and its
binding to cellular proteins was initially thought to
be responsible for cell death. A competing
hypothesis was introduced that questioned the
relevance of protein binding and instead suggested
that P450-derived oxidant stress and lipid
peroxidation causes acetaminophen-induced liver
injury (Jaeschke and Ramachandran, 2018).
Hepatotoxicity can occur because the metabolite n-
acetyl-p-benzoquinoneamine (NAPQI) is reactive
(Brune et al., 2015), and interact with covalent liver
macromolecule in the cysteine resulting in the onset
of oxidative stress (Vakiloddin et al., 2015).
Acetaminophen is activated by the enzyme
cytochrome P450 become metabolite N-acetyl-p-
benzoquinone imine (NAPQI) that suppress reactive
glutathione covalent bonds and liver with protein
(Walubo et al., 2004). This bond-related to the
toxicity of acetaminophen which causes liver injury
(Salhanick et al., 2006).
The liver injury can occur by structural damage
and seen from the histological profile of the liver
microscopically. Biochemical parameters in the
blood serum also can be used as an indicator when
the liver injury by an enzyme released from the
hepatic cell organelle into the blood (Amin et al.,
2010). Specific enzymes that indicate liver damage
is ALT (alanine aminotransferase), AST (aspartate
transaminase), TB (total bilirubin), ALP (alkaline
phosphatase) (Gowda et al., 2009; Limdi and Hyde,
66
Sasongko, H., Pratiwi, D., Amartiwi, T., Efendi, N. and Sugiyarto, .
Hepatoprotective Effect of Mountain Papaya (Vasconcellea pubescens A.DC.) Fruit Extract against Acetaminophen-Induced Acute Liver Damage.
DOI: 10.5220/0008239500660070
In Proceedings of the 1st Muhammadiyah International Conference on Health and Pharmaceutical Development (MICH-PhD 2018), pages 66-70
ISBN: 978-989-758-349-0
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2003). Liver damage can be treated with a
hepatoprotector compound (Pradhan and Girish,
2006). Many studies associate the effects of
antioxidants with hepatoprotection consequence
(Hsiao et al., 2003; Huang et al., 2010; Tzankova et
al., 2017).
Mountain papaya (Vasconcellea pubescens
A.DC.), also called Carica pubescens, are
commonly found in the Dieng plateau, Central Java
(Sasongko et al., 2016). Mountain papayas contain
flavonoid and phenolic compounds that have
antioxidant activity (Simirgiotis et al., 2009; Uribe
et al., 2015). It has antioxidant activity with IC50
value of 8,843 to 0,983 mg/100 mL (Laily et al.,
2012). This research aims to know the
hepatoprotective effect of mountain papaya fruit
extract against acetaminophen-induced acute liver
damage.
2 MATERIALS AND METHOD
2.1 Materials
The fruit of mountain papaya (Vasconcellea
pubescens A.DC) was collected from the Dieng
Plateau, Central Java, Indonesia. Studies were
carried out using male Wistar albino rats (150-250
g). Rats were obtained and all handling procedures
havc been approved by the ethics committee of the
Faculty of Medicine, Universitas Sebelas Maret with
the number 421/V/HREC/2017. Chemical like
Alanine Aminotransferase (ALT), Aspartate
Aminotransferase (AST), alkaline phosphatase
(ALP), total bilirubin were analyzed using reagent
kits (DiaSys Diagnostic, Holzheim, Germany). All
other reagents were of analytical grade. The
instruments used were freeze dryer (VirTis
BenchTop ®) and Spektrofotometer UV-Vis (Micro
Lab 300 ®).
2.2 Sample and Extract Preparation
Mountain papaya fruit with yellowish green color
was washed and cut into small pieces. The fruit was
dried using a freeze dryer. After drying, the fruit was
ground and sieved to a uniform particle size as
sample. The sample was extracted using the
maceration method with 70% ethanol solvent for
seven days. The ratio of sample to solvent was 1:10.
The filtrate of the maceration was collected and
concentrated with a rotary evaporator at 50°C to
obtain an extract.
2.3 Phytochemical Screening
Phytochemical screening was carried out to screen
class of phytoconstituents present in the ethanolic
extract of mountain papaya stem using standard
methods reported in Harborne (2012).
2.4 Animal Experimental Design
The experimental animals were acclimatized for one
week. The rats were given aquadest drink and
standard feed. The animal were divided into six
groups with group I not administered with any
treatment, group II administered with suspension of
0.25% CMC-Na as negative control, group III was
administered with suspension of silymarin as
positive control. Groups IV, V, and VI were given
mountain papaya fruit ethanolic extract (MPFE)
with variation doses 120; 240 and 480 mg/kg body
weight for 14 days. At day 14th all groups except the
normal group were induced with acetaminophen in
toxic dose (2 g/kg body weight). After 48 hours
induction of acetaminophen, blood sample was
collected to measure ALT, AST, ALP and total
bilirubin value. The liver was taken to be analysed
for histology profile.
2.5 Histopathological Studies
Liver tissues were fixed in 10% formalin for at least
24 hours, embedded in paraffin and cut into 5 µm
thick sections in a rotary microtome. The sections
were stained with hematoxylin-eosin dye and
observed under a microscope to detect
histopathological changes in the liver (Huang et al.,
2010).
2.6 Data Analysis
The analysis was performed statistically. The
normality test used was the Shapiro-Wilk test and
variance test was done by Homogeneity of variance
test. The normally and homogeneously distributed
data were analyzed by One-way Analysis of
Variance (ANOVA). To know the differences
between each treatment groups and then continued
with Bonferroni Post Hoc test.
Hepatoprotective Effect of Mountain Papaya (Vasconcellea pubescens A.DC.) Fruit Extract against Acetaminophen-Induced Acute Liver
Damage
67
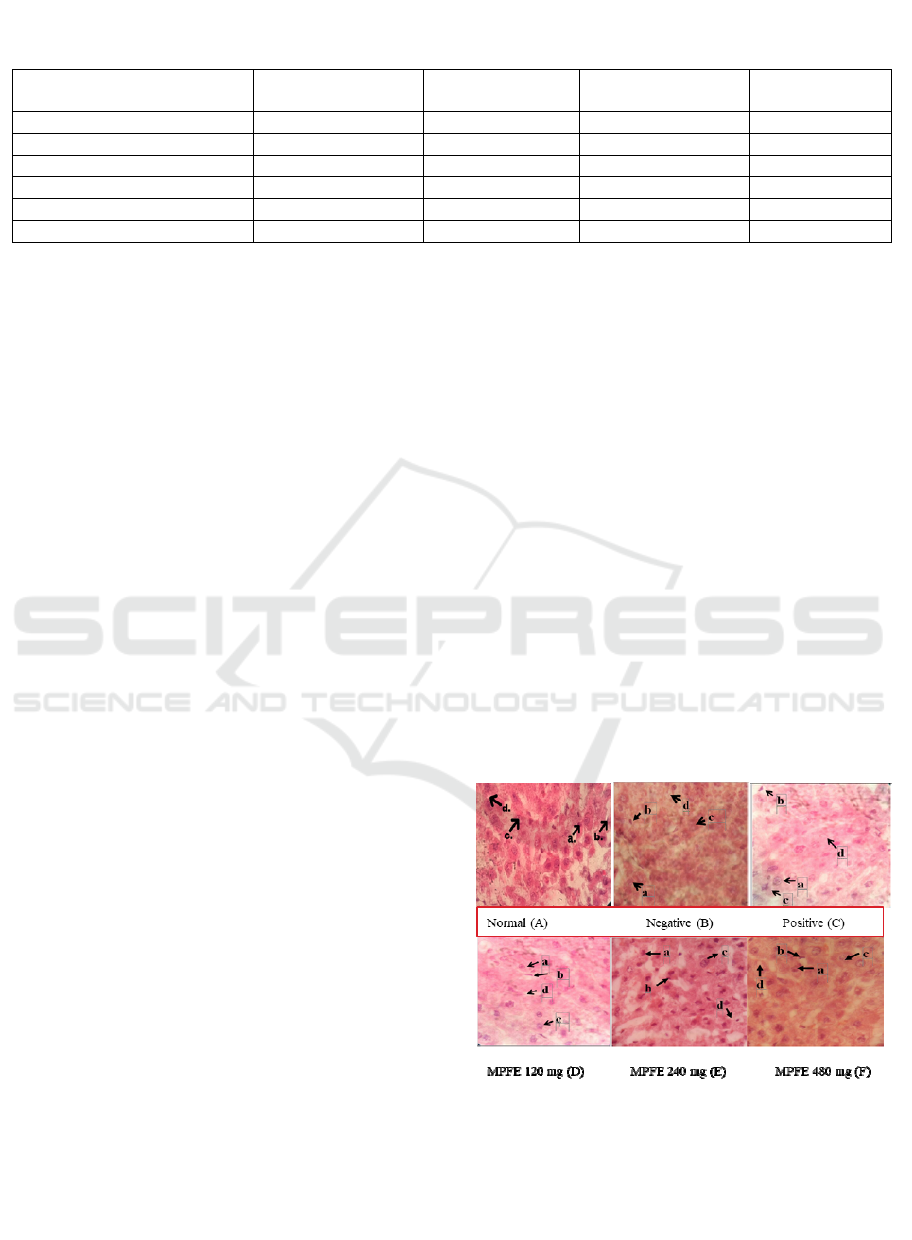
Table 1: The effect of mountain papaya (Vasconcellea pubescens A.DC.) fruit extract against acetaminophen-induced
on biochemical parameters.
Groups ALT
(m
g
/ dl)
AST
(m
g
/ dl)
ALP
(m
g
/ dl)
TB
(m
g
/ dl)
Normal control
51.66 ± 5.25* 223.54 ± 14.67*
548,70 ± 73,72
0,502 ± 0, 01
Ne
g
ative control
461.36 ± 16.60 894.72 ± 22.46
774,38 ± 25,95
0,584 ± 0,02
Silymarin 100 mg/kg B.W
76.24 ± 13.54* 216.68 ± 12.33*
564,92 ± 26,84*
0,512 ± 0,02
Extract dose120 mg/kg B.W
284.23 ± 17.20* 355.68 ± 19.80*
612,60 ± 20,22
0,538 ± 0,02
Extract dose 240 mg/kg B.W
255.96 ± 14.10* 291.56 ± 19.26*
589,72 ± 18,76
0,534 ± 0,08
Extract dose 480 m
g
/k
g
B.W
155.7 ± 15.84* 245.24 ± 18.25*
573,80 ± 12,19
0,518 ± 0,01
Symbols represent statistical significance. *p < 0.05, as compared to negative control group. n = 5 animals in each
group
3 RESULT AND DISCUSSION
3.1 Phytochemical Screening
The phytochemical compound of mountain papaya
(Vasconcellea pubescens A.DC) ethanolic extract
showed flavonoids, tannins and phenolic.
3.2 Hepatoprotective Effect
The rats’ biochemical parameter result like ALT,
AST, ALP and total bilirubin are shown in Table I.
The results demonstrated that ALT and AST were
found to be significantly increased in rats treated
with acetaminophen when compared with the
negative control group (P<0.05) but not significantly
on ALP and total bilirubin serum. The
administration of mountain papaya extract for 14
days significantly decreased the activity of serum
alanine aminotransferase and serum aspartate
transaminase in a dose-dependent manner in
acetaminophen-induced liver damage in rats
compared to that of the hepatotoxic group
(acetaminophen treatment) (P<0.05). The serum
level of ALT and AST are largely used for
determination of liver damage (Nurrochmad et al.,
2013). Serum glutamic pyruvic transaminase
(SGPT) or also called ALT (alanine
aminotransferase) is a specific enzyme that can
estimate the damage of a cell especially in the liver
(Gowda et al., 2009; Limdi and Hyde, 2003).
The mechanism of hepatotoxic from
acetaminophen is caused by the damage of hepatic
cell resulting from metabolites formed at the time of
reaction with cytochrome P450. In therapeutic dose,
the main metabolic pathway of acetaminophen is
through glucuronidation and sulfation in the liver,
and only slightly metabolized by the P450
cytochrome which produces N-acetyl quinone imine
(NAPQI). NAPQI in such amounts can be detoxified
by conjugation with glutathione (GSH). While
paracetamol is in excessive doses, it causes
saturation of the sulfate pathway, resulting in large
NAPQI formation and GSH depletion (Li et al.,
1994). Reduced amounts of glutathione will lead the
formation of Reactive Oxygen Species (ROS) and
Reactive Nitrogen Species (RNS) that cause necrosis
of hepatocytes. The presence of ROS will lead to a
loss of mitochondrial potential membrane and loss
of mitochondrial ability in synthesizing ATP. The
loss of ATP will lead the present of necrosis (Hinson
et al., 2010).
3.3 Histopathological Profile
Use the differences microscopic appearance of
hepatic cells between treatment groups with ethanol
extract of mountain papaya can be seen in Figure 1.
Figure 1. Histologic Profile of the Rat’s Liver.
Description: (A) normal Group, (B) negative control, (C)
positive control, (D) MPFE 120 mg/kg b.w, (E) MPFE 240
mg/kg b.w, (D) MPFE 480 mg/kg b.w; (a) Normal cells,
(b) Picnotic, (c) Cariorixis, (d) Kariyolysis
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
68

From the histological profile that showed the
condition of the hepatic cell in the treatment group
compared with the negative group, there were cells
still in normal condition, but some cells have been
damaged (necrotic). Early morphological changes
include cytoplasmic edema, dilatation of the
endoplasmic reticulum and polysomal
disaggregation. The next process occurs triglyceride
accumulation as fatty grains in the cell, progressive
mitochondrial swelling with damaging crystals and
complex biochemical swelling. The next stages may
experience hydropic degeneration, separation of cell
structures, pythonic cell nuclei, cariorixis,
karyolysis, breakage of the plasma membrane, and
eventually necrosis (Kumar et al., 2017). The dead
nucleus will shrink, have irregular and dark
boundaries. This process is called piknosis, and its
core is called piknotik. The other possibility, the
nucleus may be destroyed, leaving fragments of
chromatinic substances that is dispersed in cells
called cariorecidal. Finally, in a certain condition,
the dead nucleus loses the ability to absorb the dye
again and completely disappears, a process called
karyolysis (Wilson and Price, 2006).
4 CONCLUSIONS
The results showed that mountain papaya
(Vasconcellea pubescens A.DC) could significantly
decrease liver cell injury (p <0.05) by ALT, AST
and liver histology profile parameters in which each
dose has the same ability.
ACKNOWLEDGEMENTS
The author would like to thank Universitas Sebelas
Maret that funded this research with the Hibah
PKLP PNBP Grants scheme.
REFERENCES
Amin, K.A., Abdel Hameid, H., Abd Elsttar, A.H., 2010.
Effect of food azo dyes tartrazine and carmoisine on
biochemical parameters related to renal, hepatic
function and oxidative stress biomarkers in young
male rats. Food Chem. Toxicol. 48, 2994–2999.
https://doi.org/10.1016/j.fct.2010.07.039
Brune, K., Renner, B., Tiegs, G., 2015.
Acetaminophen/paracetamol: A history of errors,
failures and false decisions. Eur. J. Pain 19, 953–965.
https://doi.org/10.1002/ejp.621
Gowda, S., Desai, P.B., Hull, V.V., Math, A.A.K.,
Vernekar, S.N., Kulkarni, S.S., 2009. A review on
laboratory liver function tests. Pan Afr. Med. J. 3.
Harborne, J.B., 2012. Phytochemical Methods: A Guide to
Modern Techniques of Plant Analysis. Springer
Science & Business Media.
Hinson, J.A., Roberts, D.W., James, L.P., 2010.
Mechanisms of Acetaminophen-Induced Liver
Necrosis, in: Adverse Drug Reactions, Handbook of
Experimental Pharmacology. Springer, Berlin,
Heidelberg, pp. 369–405.
Hsiao, G., Shen, M.-Y., Lin, K.-H., Lan, M.-H., Wu, L.-
Y., Chou, D.-S., Lin, C.-H., Su, C.-H., Sheu, J.-R.,
2003. Antioxidative and Hepatoprotective Effects of
Antrodia camphorata Extract. J. Agric. Food Chem.
51, 3302–3308. https://doi.org/10.1021/jf021159t
Huang, B., Ban, X., He, J., Tong, J., Tian, J., Wang, Y.,
2010. Hepatoprotective and antioxidant activity of
ethanolic extracts of edible lotus (Nelumbo nucifera
Gaertn.) leaves. Food Chem. 120, 873–878.
https://doi.org/10.1016/j.foodchem.2009.11.020
Jaeschke, H., Ramachandran, A., 2018. Oxidant Stress and
Lipid Peroxidation in Acetaminophen Hepatotoxicity.
React. Oxyg. Species Apex NC 5, 145–158.
Kumar, K.E., Harsha, K., Sudheer, V., others, 2013. In
vitro antioxidant activity and in vivo hepatoprotective
activity of aqueous extract of Allium cepa bulb in
ethanol induced liver damage in Wistar rats. Food Sci.
Hum. Wellness 2, 132–138.
Kumar, V., Abbas, A.K., Aster, J.C., 2017. Robbins Basic
Pathology E-Book. Elsevier Health Sciences.
Laily, A.N., Suranto, S., Sugiyarto, S., 2012.
Characterization of Carica pubescens in Dieng
Plateau, Central Java based on morphological
characters, antioxidant capacity, and protein banding
pattern. Nusant. Biosci. 4.
Larsen, F.S., Wendon, J., 2014. Understanding
paracetamol-induced liver failure. Intensive Care Med.
40, 888–890. https://doi.org/10.1007/s00134-014-
3293-9
Li, Y., Wang, E., Patten, C.J., Chen, L., Yang, C.S., 1994.
Effects of flavonoids on cytochrome P450-dependent
acetaminophen metabolism in rats and human liver
microsomes. Drug Metab. Dispos. 22, 566–571.
Limdi, J.K., Hyde, G.M., 2003. Evaluation of abnormal
liver function tests. Postgrad. Med. J. 79, 307–312.
https://doi.org/10.1136/pmj.79.932.307
Muriel, P., 2017. Liver Pathophysiology: Therapies and
Antioxidants. Academic Press.
Nurrochmad, A., Margono, S.A., Sardjiman, Hakim, A.R.,
Ernawati, Kurniawati, E., Fatmawati, E., 2013.
Hepatoprotective and antioxidant activity of
pentagamavunon-0 against carbon tetrachloride-
induced hepatic injury in rats. Asian Pac. J. Trop.
Med. 6, 438–442. https://doi.org/10.1016/S1995-
7645(13)60070-X
Pradhan, S.C., Girish, C., 2006. Hepatoprotective herbal
drug, silymarin from experimental pharmacology to
clinical medicine. Indian J. Med. Res. 124, 491.
Hepatoprotective Effect of Mountain Papaya (Vasconcellea pubescens A.DC.) Fruit Extract against Acetaminophen-Induced Acute Liver
Damage
69

Salhanick, S.D., Belikoff, B., Orlow, D., Holt, D.,
Reenstra, W., Buras, J.A., 2006. Hyperbaric Oxygen
Reduces Acetaminophen Toxicity and Increases HIF-
1α Expression. Acad. Emerg. Med. 13, 707–714.
https://doi.org/10.1197/j.aem.2006.01.029
Sasongko, H., Sugiyarto, S., Efendi, N.R., Pratiwi, D.,
Setyawan, A.D., Widiyani, T., 2016. Analgesic
Activity of Ethanolic Extracts of Karika Leaves
(Carica pubescens) In Vivo. J. Pharm. Sci. Clin. Res.
1, 83–89. https://doi.org/10.20961/jpscr.v1i2.1938
Simirgiotis, M.J., Caligari, P.D.S., Schmeda-Hirschmann,
G., 2009. Identification of phenolic compounds from
the fruits of the mountain papaya Vasconcellea
pubescens A. DC. grown in Chile by liquid
chromatography–UV detection–mass spectrometry.
Food Chem. 115, 775–784.
https://doi.org/10.1016/j.foodchem.2008.12.071
Tzankova, V., Aluani, D., Kondeva-Burdina, M.,
Yordanov, Y., Odzhakov, F., Apostolov, A.,
Yoncheva, K., 2017. Hepatoprotective and antioxidant
activity of quercetin loaded chitosan/alginate particles
in vitro and in vivo in a model of paracetamol-induced
toxicity. Biomed. Pharmacother. 92, 569–579.
https://doi.org/10.1016/j.biopha.2017.05.008
Uribe, E., Delgadillo, A., Giovagnoli-Vicuña, C., Quispe-
Fuentes, I., Zura-Bravo, L., 2015. Extraction
Techniques for Bioactive Compounds and Antioxidant
Capacity Determination of Chilean Papaya
(Vasconcellea pubescens) Fruit [WWW Document]. J.
Chem. URL
https://www.hindawi.com/journals/jchem/2015/34753
2/abs/ (accessed 2.17.18).
Vakiloddin, S., Fuloria, N., Fuloria, S., Dhanaraj, S.A.,
Balaji, K., Karupiah, S., 2015. Evidences of
hepatoprotective and antioxidant effect of Citrullus
colocynthis fruits in paracetamol induced
hepatotoxicity. Pak. J. Pharm. Sci. 28, 951–957.
Walubo, A., Barr, S., Abraham, A.M., Coetsee, C., 2004.
The role of cytochrome–P450 inhibitors in the
prevention of hepatotoxicity after paracetamol
overdose in rats. Hum. Exp. Toxicol. 23, 49–54.
https://doi.org/10.1191/0960327104ht415oa
Wilson, L.M., Price, S.A., 2006. Patofisiologi konsep
klinis proses-proses penyakit.. EGC Jakarta.
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
70
