
Formulation of Sechium edule Extract Effervescent Granule with the
Variation of Citric Acid, Tartrate Acid and Sodium Bicarbonate
Ina Ba’dia Grajang and Iis Wahyuningsih
Faculty of Pharmacy, Universitas Ahmad Dahlan, Jl. Prof. Dr. Soepomo, Janturan , Kota Yogyakarta, Indonesia
Keywords: Effervescent granules, extract, Sechium edule.
Abstract: The Sechium edule fruit is traditionally proven to contain antiulcer. Thus, it is necessary to develop dosage
formulations. An effervescent granule is chosen as an alternative form of drug delivery, considering its
dosage preparation may apply simply within a short amount of time. This study aims to discover the
composition of the mixture of citric acid, tartrate acid, and sodium bicarbonate met the physical test
requirement for effervescent granule. Effervescent granules are made by wet granulation technique (the
mixture of alkali-acid) by <25% of relative humidity on 20-25
o
C temperature. There are three effervescent
granule formulas, consisting a different portions composition of citric acid, tartrate acid, and sodium
bicarbonate: formula I = (2.5 :5:15); formula II = (2.25:4.5:16.88); and formula III = (2:4:18). Physical test
on granules consists of flow rate test, moisture content, time of dissolution, pH and organoleptic. Following
trials performed on Formulas I, II, and III of effervescent granule, the granules flow time of each formula
are:1.48;1.53;1.48 seconds moisture content: 3.40; 3.62; 3.50%, time of solubility : 4.37; 4.25; 3.65 minutes,
pH: 6.62; 6.54; 6.59, and likeness survey: (Colour=1.93; 1.90;2,0.Scent=2.0; 1.93; 2.07. Flavour=1.76; 2.06;
2.13). Those three formulas met the good physical test requirement, except on the moisture content.
Formula III go the most preference on colour, scent and taste.
1 INTRODUCTION
Sechium edule is one of the plants used for
traditional medicine. Sechium edule has many
potentials as a drug to treat some diseases; one of
them is as an antiulcer (Kamble et al., 2008). The
fruits and leaves of Sechium edule contain saponins
and flavonoids. Besides that, the fruit also contains
alkaloids and tannins, while the leaves contain
flavonoids and polyphenols (Gandhu et al., 2012).
The fruit also contains saponins, alkaloids and
tannins, while the leaves contain saponins,
flavonoids and polyphenols (Pratiwi, 2011). The
active ingredients in the contents of Sechium edule
that function as antiulcer are the tannin, flavonoid,
and alkaloid compounds contained in the skin and
fruit of Sechium edule (Hagerman, 2000).
In Rofifah's research (2016), the extract of the
Sechium edule has antiulcer effect at the dose of 300
mg/kg BW when administered to a rat. Converted
into a human the dose, it equals 3.360 mg for single
use. With such a large dose of use, it is necessary to
develop a formulation to produce an appropriate
dosage form acceptable to the patient. Effervescent
granules are chosen as an alternative dosage form
because of the advantages of these dosage forms.
First, they are easy to use; the preparation of the
solution with the right dosage of the drug can be
done in fast, convenient time. Second, it can provide
a refreshing delicious sensation because of
carbonate, which can help improve the taste (Allen,
2002).
Effervescent granule processing is generally
derived from the acid combination of citric acid-
tartaric acid rather than just one acid because the use
of single acid alone would be difficult. When using
only citric acid, it will produce a mixture of
adhesives and difficult for granulation. When only
tartrate acid is used, the resulting granule will easily
lose its strength, easy to clot and will produce a fast
effervescent reaction (Ansel, 1989). Sodium
bicarbonate is used as the alkali reaction and acts to
neutralize the citric acid and tartaric acid and can
produce foam and liberate carbon dioxide gas and is
completely soluble in water (Pulungan et al., 2004).
The acid mixture (citric acid-tartaric acid) and alkali
(sodium bicarbonate) in effervescent granule extract
of the Sechium edule aims to provide sparkle or taste
54
Grajang, I. and Wahyuningsih, I.
Formulation of Sechium edule Extract Effervescent Granule with the Variation of Citric Acid, Tartrate Acid and Sodium Bicarbonate.
DOI: 10.5220/0008239300540060
In Proceedings of the 1st Muhammadiyah International Conference on Health and Pharmaceutical Development (MICH-PhD 2018), pages 54-60
ISBN: 978-989-758-349-0
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

effects such as soda drinks that take place quite
rapidly and produce a clear solution (Pulungan et al.,
2004). Therefore, the drug given in the form of an
effervescent granule with a mixture of citric acid,
tartaric acid and sodium bicarbonate will provide a
refreshingly delicious taste sensation (Ansel, 1989).
The objective of this research is to find out the
composition of citric acid, tartrate, and sodium
bicarbonate mixture which meets the physical
requirement of effervescent granule extract of
squash fruit (Sechium edule).
2 MATERIALS AND METHOD
2.1 Materials
The materials used in this research are Sechium
edule obtained from farmers’ group of Mount
Sindoro, Parakan, Temanggung, Central Java,
ethanol 96%, ethanol 70%, HCl 2 N, ammonia,
Mayer reagent, FeCl
3
, citric acid, tartaric acid,
sodium bicarbonate, lactose, aspartame, and PVP.
2.2 Methods
2.2.1 Preparation of Sechium edule Extract
One kilogram of Sechium edule crude simplicia
granules was performed by maceration using 96%
ethanol solvent of 5 L stirred with intermittent
shaking for 3 hours at 400 rpm for 72 hours (3 days).
The maceration results are evaporated over the
waterbath at 50-60°C until a thickened extract is
obtained, then placed in a dark container and stored
in a cool place for further use. The evaluations of the
extract of the Sechium edule include:
• Calculation of yield of the extract: the extract
yield of the Sechium edule is calculated by
comparing the initial weight of the simplisia
and the final weight of the extract produced.
• Organoleptic extract of Sechium edule:
organoleptic test includes the observation of
colour, smell, taste and consistency of the
extract.
• The moisture content of Sechium edule
extract: the moisture content of the extract was
tested using a Halogen Moisture Analyzer
with heating at 105
o
C for 15 minutes, the
percentage content of the sample will be
automatically listed. Good moisture content
does not exceed 10% (Depkes RI, 2000).
• Identification of flavonoids, alkaloids, and
tannins: this identification was performed by
tube test method using the appropriate
reagents for the class of compounds to be
tested i.e. flavonoids, alkaloids, and tannins.
Ammonia vapour reagents are used for the
examination of flavonoids (Robinson, 1995).
Mayer reagent is used to detect alkaloid
compounds and then observe the presence or
absence of precipitation (Mojab et al., 2003).
The FeCl
3
reactor is used for the examination
of polyphenols (tannins) by observing the
colour of the result solution (Sri et al., 2014).
2.2.2 Making Effervescent Sechium edule
Granule
Effervescent granules were made using a wet
granulation method. Separated between acidic
components and alkalic components. Effervescent
granules are made under special conditions of 25%
relative humidity at 20-25°C (Siregar, 2007). All
ingredients on the formula were sieved with a sieve
mesh size 50 and dried earlier in the oven for 1 hour
then weighed according to the formula. The acid
mixture consisted of dry extract of Sechium edule,
citric acid, tartaric acid, aspartame, lactose and PVP,
dripped with 96% ethanol to form a mass that can be
clenched, sieved with sieve mesh size 14, dried in an
oven at 50 °C for 3 hours. Dry granules are sifted
back with sieve mesh size 16. The alkalic
components (Sodium bicarbonate mixed with
residual lactose, and residual PVP) were dripped
with 96% ethanol to form a mass that can be
clenched, sieved with mesh no. 14, dried in an oven
at 50 ° C for 3 hours. Dry granules are sieved back
with mesh sieves size 16. Acid components and
alkalic components are mixed in special room
conditions (temperature 20-25
0
C with 25% relative
humidity (RH)) until they became homogeneous.
The mixture was packed in an airtight container. The
granules obtained were evaluated. The organoleptic
examination includes examination of colour, flavour
and taste. The formulation of granule can be seen in
Table 1.
Formulation of Sechium edule Extract Effervescent Granule with the Variation of Citric Acid, Tartrate Acid and Sodium Bicarbonate
55
2.2.3 Physical Characteristics of Effervescent
Granules Abstract
• Effervescent granule flow time test
Weighed a total of 50 g, mass of granules were
inserted into the flowmeter. The time required for
the granules to flow through the lid of the funnel
opening was recorded. Testing was done five times.
Good flow time requirement <10 g/ sec (Voigt,
1995).
• Effervescent granule moisture content
Five gram mass of granules inserted into the
moisture balance tool at 105
0
C for 15 minutes. After
15 minutes, the percentages (%) of water levels will
automatically displayed on the tool display. The
requirements of good effervescent moisture content
granules levels are 0.4-0.7% (Fausett et al., 2000).
• Soluble time of effervescent granule
The effervescent granules weighed 5 g was then
inserted into 200 mL of water. Time recording is
required until the granules dissolve. Requirements
for good effervescent granule time of less than 5
minutes result in a clear solution (Siregar, 2007).
• pH of granule effervescent
The effervescent solution was prepared by weighing
5 g of mass of granules dissolved in 200 mL of
water. pH meter calibration was performed by first
using buffer solution of pH 4.0 and pH 7.0. Once
calibrated, the electric pH meter was dipped into
effervescent solution that no longer have gas
bubbles. the pH value obtained was noted. The pH
of effervescent solution is said to be good if the pH
is close to neutral i.e. 6-7 (Widayanti et al., 2012).
• Hedonic test
Hedonic test was administered to 30 panel lists age
17 years and above, healthy and had no disorder
around the mouth that can affect the taste. They had
been instructed not to consume food or drink prior to
the test that could affect the assessment. They were
asked to taste and assess the taste, smell and colour
of the 5 g effervescent granule samples which had
been diluted with 200 mL water. The panellists were
expected to fill in the provided questionnaires.
2.3 Data Analysis
The result of granule physical characteristics test
was statistically analyzed using one way ANOVA.
This was followed by LSD test with 95% confidence
level to know the significant difference between test
result formula.
3 RESULT AND DISCUSSION
3.1. Simplicia and Sechium edule Extract
The Sechium edule are dried in the sun for 1 day and
continued to be dried in the oven at 50°C for 2 days
to reduce the moisture content. The use of the oven
as a dryer means that the dried Sechium edule are
evenly dried and the drying time is faster because it
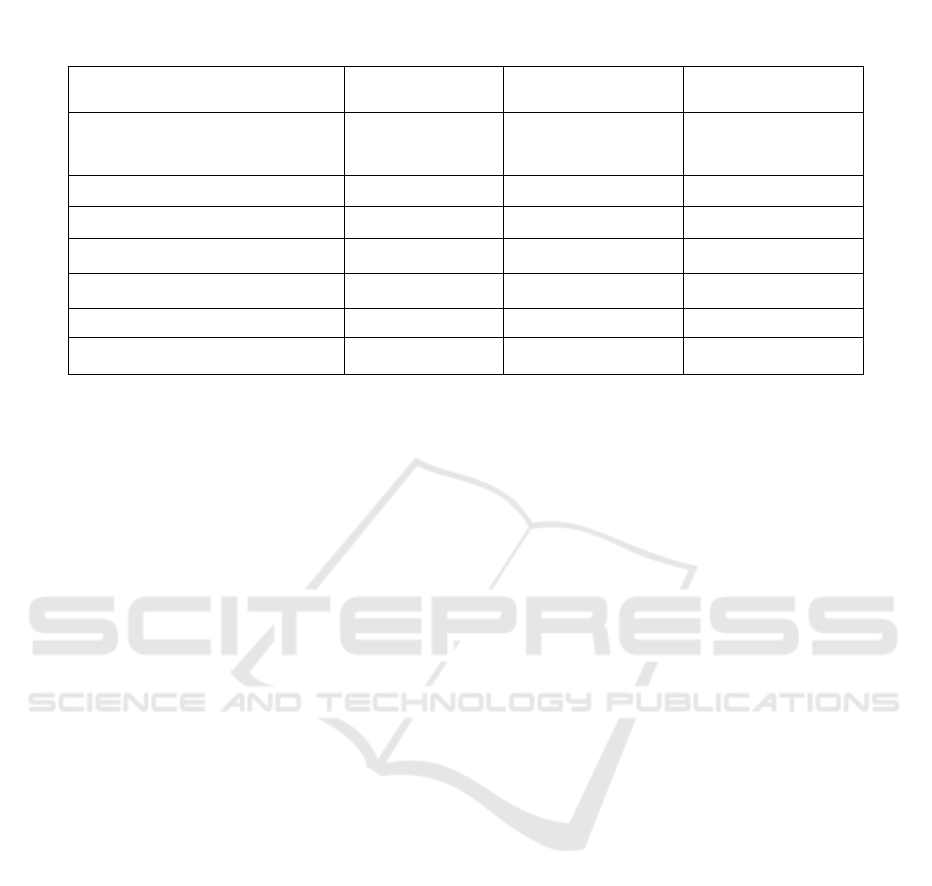
Table 1: Effervescent granule formulation of Sechium edule (@ 50 g)
Ingredients Formula I (g) Formula II (g) Formula III (g)
Dried Sechium edule extract
(Viscousextract:Lactose (1: 5))
20.16 20.16 20.16
Citric Acid 2.5 2.25 2
Tartaric acid 5 4.5 4
Sodium bicarbonate 15 16.88 18
Polyvinyl pyrrolidone (PVP) 1.5 1.5 1.5
Aspartame 2 2 2
Lactose ad 50 g 3.84 5.43 4.68
Note :
F I: Mixtures Citric acid, tartaric acid, and sodium bicarbonate: (2.5 g: 5 g: 15 g)
F II: Mixture Citric acid, tartaric acid, and sodium bicarbonate: (2.25 g: 4.5 g: 16.88 g)
F III: Mixture Citric acid, tartaric acid, and sodium bicarbonate: (2 g: 4 g: 18 g)
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
56

is not affected by weather conditions and protected
from UV damage (Sulistyani and Eka, 2011). The
powder of simplicia obtained from 20 Kg of squash
is 1000 g of crude simplicia powder.
The Sechium edule extract is prepared by
maseration method. The solvent used in this study
was 96% ethanol because the content of the active
substance of the Sechium edule had a good solubility
in ethanol (Firdous et al., 2012). The maceration was
performed with a ratio of 1000 g of simplicia of
Sechium edule and 5 L of ethanol 96% per 1 time
maceration. From the extract that was obtained thick
extract 194.626 g from 1000 g of simplicia with
yield of extract 19.463%. The result of organoleptic
examination of Sechium edule extract is dark
brownish green colour extract, unique smell of
aromatic extract, bitter extract flavour and slightly
thickened extract form.
The identification of the compounds contained in
the extract of the Sechium edule is done by tube test.
The tests include flavonoids, alkaloids, and tannins.
Flavonoid test obtained positive results by passing
the extract that has been dripped on filter paper with
ammonia vapour. Filter paper turns yellow due to
flavonoid reaction with ammonia vapour to form salt
and a kinoid structure on ring B that will create
double bonds that conjugated longer so that it will
increase the colour intensity (Robinson, 1995). In
the alkaloid test, positive result of alkaloid with
Mayer’s reagent was characterized by turbidity and
formed a little white precipitate. The nitrogen in the
alkaloids reacts with the K
+
metal ions from the
tetraiodomerkurat (II) potassium to form the
potassium-alkaloid complex precipitation (Marliana,
2005). In the tannin test, a positive result was
obtained by adding the FeCl
3
reagent to form a
blackish-green colour. The formation of blackish-
green colour on the extract after being added with
FeCl
3
is because tannin will form complex
compounds with Fe
3+
ions (Mangunwardoyo et al.,
2008).
3.2. Granulation
Formulation of effervescent granule extract of
Sechium edule with a mixture of citric acid, tartaric
acid, sodium bicarbonate and other excipients of the
three formulas is a modified obtained from the trial
results. Manufacture of effervescent granules with
wet granulation method has the advantage of making
a simple, quick and homogeneous granule produced
(Parikh, 2005). Effervescent granules are prepared
by separating the acid and the alkali components to
prevent reaction between acid-base components
when mixed in wet conditions (Parikh, 2005). Citric
acid and tartaric acid act as the source of acid,
sodium bicarbonate as the alkali source, PVP as the
binder, aspartame as the sweetener, and lactose as
the filler. All the processes were performed; both
formulations and physical properties of effervescent
granules were performed in a special room with RH
of 25% and temperature of 20-25
o
C (Siregar, 2007).
3.3. Physical Properties of Effervescent
Granule Extract of Sechium edule
Effervescent granules were tested for their physical
properties using methods such as flow time test,
moisture content test, soluble test, pH test, and
Hedonic test. The test results can be seen in Table 2.
The flow time of effervescent granule extract of
Sechium edule from F I is shorter compared to F II
and F III. This is due to F I that produced
effervescent granules with larger particle size
compared to other formulas (determined based on
visual observations since they are not measured).
Table 2: Physical properties of granul effevescent extract of Sechium edule
No Test Parameter
Value (X ± SD)
Formula I Formula II Formula III
1 Granule flow time (seconds)
1.47±0.20 1.53± 0.14 1.48 ± 0.10
2 Granule water content (%)
3.40 ± 0.34 3.62 ± 0.44 3.50 ± 0.63
3 Granule soluble time (minutes)
4.37 ± 0.12 4.25 ± 0.08 3.65±0.55*
4 pH value
6.62 ± 0.04 6.54 ± 0.08 6.59 ± 0.05
5 Hedonic test
Colour
1.93 ± 0.78 1.90 ± 0.71 2.00 ± 0.69
Scent
2.00 ± 0.79 1.93 ± 0.74 2.07 ± 0.91
Taste
1.77 ± 0.77 2.07 ± 0.74 2.13 ± 0.63
Description: * There is a difference with FI
Formulation of Sechium edule Extract Effervescent Granule with the Variation of Citric Acid, Tartrate Acid and Sodium Bicarbonate
57

According to Ansel (1989), one molecule of water
per citrate acid molecule determines powder
formation, whereas according to Voigt (1984),
enlargement of particle size can generally improve
flow characteristic or powder glide power.
Differences in the concentration of citric acid and
tartaric acid (1:2) used can affect the effervescent
granular flow characteristic. The higher the acid
concentration used, the better the flow characteristic
(Widayanti et al., 2012). The flow time is influenced
by the shape, size, porosity, density, and particle
frictional forces and experimental conditions.
Tartaric acid has a greater density than citric acid so
that granules containing tartrate acid will have
greater density. Large densities show large
molecular weights that will flow more easily due to
greater gravity (Anshory et al., 2007). According to
Mohrle (1980), citric acid has a specific density
value of 1.665 mg/mL, while tartaric acid has a
specific density value of 1.7598 mg/mL. The
statistical analysis of the three distributed formulas
is 0.930 and the homogeneous data is 0.086. The
result of statistical test shows that the three
effervescent granule formulations that have been
made have an average granule flow time which is
not significantly different and less than 10 seconds.
These results show that citric acid, tartaric acid, and
sodium bicarbonate in the formula have no
significant effect on granule flow time.
The moisture content of FI, FII, and FIII of
effervescent granules in this study has not fulfilled
the requirements of good effervescent granule water
content i.e. 0.4-0.7% (Fausett et al., 2000). The
moisture content was not achieved because of the
limitations in the room where the effervescent
produced has high relative humidity, causing the raw
material from the effervescent to react quickly.
Despite efforts to reduce the relative humidity of the
room, room control can only reach 29% with a
temperature of 29
o
C, whereas the room relative
humidity for the preparation of effervescent
preparations is 25% with a temperature of 20-25
o
C
(Siregar, 2007).
This limitation makes the granules absorb
moisture from the environment so that moisture
content in the effervescent granule becomes high.
Although the effervescent granule materials have
been dried in the oven, effervescent granules
produced cannot reach moist moisture of 0.4-0.7%.
It is possible to achieve moisture content balance
between the effervescent granule materials and the
humidity of the manufacturing chamber (Budi and
Lisa, 2007). The non-fulfilment of the effervescent
granular moisture content will affect the flow
characteristic and the dissolution rate (Widayanti et
al., 2012). The ability of sodium bicarbonate to
absorb moisture during storage is less than citric
acid and tartaric acid, so the moisture contained in
sodium bicarbonate is less than the moisture
contained in citric acid and tartaric acid. Thus, the
greater the amount of sodium bicarbonate used in
the production of effervescent granules will further
decrease the moisture content of effervescent
granules produced.
The nature of citric acid that is hygroscopic also
has the potential to absorb water vapour in the air.
Sodium bicarbonate is not hygroscopic and at room
temperature that has a moist content of less than 1%
(Lindberg et al., 1992). From the statistical test
obtained, the average of granule moisture content of
the three formulas is not significantly different.
The soluble time of FI, FII, and FIII of
effervescent granules in this study fulfilled the
requirement of good soluble time i.e. less than 5
minutes. The process of dissolution of effervescent
granules begins with the penetration of water into
the effervescent granules. The presence of water
penetration into the effervescent granules will
produce acid and alkali reactions that will produce
CO
2
gas. In the presence of CO
2
gas, the process of
breaking the granules will be faster and indirectly
accelerate the process of dissolving granules in
water.
The binder used in this study was PVP 3%. PVP
has hydrophilic properties that will facilitate the
penetration of water into the effervescent granules,
which will accelerate the dissolution of effervescent
granules in the water. According to Rizal and Widya
(2014), the citric acid contains water when reacting
with sodium bicarbonate containing carbon dioxide
gas then it will produce sodium citrate, water and
carbon dioxide gases three times faster that can help
solubility. This is supported by Nugroho (1999) who
states that the presence of carbon dioxide gases
produced help solubility without involving manual
stirring on condition that all components are highly
soluble in water. Based on a study conducted by
Hayu (2015), the combination of citric acid and
tartaric acid had a significant effect on the dissolving
time of the effervescent tablets made. The higher the
tartaric acid in the tablet, the higher the solubility
(quick to dissolve). This occurs because citric acid
has lower solubility than tartaric acid, and tartaric
acid can react with sodium bicarbonate which can
accelerate the solubility of effervescent tablets.
According to Mandagi et al. (2015), the greater the
concentration of sodium bicarbonate and the smaller
percentage of citric acid in the effervescent formula,
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
58

the faster the soluble time in water. Otherwise, the
less sodium bicarbonate, the greater percentage of
citric acid in the formula the longer the granule
dissolves in water. This is because sodium
bicarbonate acts as a destroyer of effervescent
granules in water so that it can dissolve completely
without stirring and when sodium bicarbonate reacts
with water it carbonates (Dwijayanti, 2009). Alkali
effect is greater than the acid mixture effect. Thus it
is predicted that the base is more dominant in
determining the effervescent granule soluble time.
Comparing soluble time between F I and F III, there
was a significant difference due to differences in the
composition of citric acid, tartaric acid and sodium
bicarbonate.
The pH test needs to be done because if the
effervescent solution that is formed is too acidic it
can irritate the stomach whereas if it has too much
alkaline it will have bitter taste and bad taste. pH
values of F I, F II, and F III of effervescent granules
in this study are qualified as good pH values,
ranging from 6.54 to 6.62. The pH of effervescent
solution is said to be good if the pH is close to
neutral i.e. 6-7 (Widayanti et al., 2012). The
statistical results show that the three formulas have
different granule pH values not significant.
The hedonic test is a test method used to measure
the level of preference of a product using a rating
sheet (Singh-Ackbarali and Maharaj, 2014).
Assessment categories consist of 4 levels namely (1)
do not like; (2) somewhat like; (3) like, and (4) like
very much. To know whether there is a difference
between treatments an analysis of variance needs to
be done seen from the value of F arithmetic
compared with the value of F table. If the value of F
arithmetic > F table then the treatment is very
different (significantly different). Considering that
the difference level is significant, the test was
continued with DMRT (Duncan Multiple Rating
Test) method to know which treatment is the same
or different (Itsagusman, 2013). The hedonic test
was seen from 3 hedonic test parameters (colour,
scent, and taste). The results of the tests indicate that
the highest hedonic average score belongs to
formula III in terms of colour (2.0), scent (2,07), and
taste (2.13). The results of the analysis of the
organoleptic colour, smell, and taste test varieties
showed no significant differences between formulas
I, II, and III. The conclusion of hedonic test for the
colours, aromas, and flavours of these three formulas
is that they have an average score of 2 which means
they are in the category of "somewhat like". This
means that based on the general panellist’s response
on the three formulas of effervescent granules they
are less attracted to the colour, the smell of
effervescent granule drink, and that the taste of
effervescent granule drink extract of Sechium edule
on the three formulas is too sweet. These results
show that required the modification of effervescent
granule formula is required to produce health drinks
that is acceptable to the people.
4 CONCLUSIONS
The formula that produces the best effervescent
granules of the best Sechium edule extract is formula
III whose composition of the mixture of citric acid,
tartaric acid, and sodium bicarbonate is 2: 4: 18.
This best formula has physical properties of flow
time of 1.48 seconds, water content of 3.50%,
soluble time 3.65 minutes, pH 6.59, and has the
highest degree of preference (colour 2.0, aroma 2.07,
and taste 2.13).
ACKNOWLEDGEMENTS
This study was supported by Kemenristek Dikti
Republik Indonesia.
REFERENCES
Allen, V.L., 2002, The Art, Science and Technology of
Pharmaceutical Compounding, 2nd Ed, 99-101,
American Pharmaceutical Assosiation, Washingtong
D.C.
Ansel, H.C., 1989, Introduction to Pharmaceutical
Dosage Form, translated by Farida Ibrahim, Pengantar
Bentuk Sediaan Farmasi, Edisi IV, Indonesia
University Press, Jakarta.
Anshory, H., Syukri, Y., dan Malasari, Y., 2007,
Formulasi Tablet Effervescent dari Ekstrak Ginseng
Jawa (Tlinum panicukatum) dengan Variasi Kadar
Pemanis Aspartam, Jurnal Ilmiah Farmasi, Vol. 4 No.
I.
Budi, A.S.L., dan Lisa, N., 2007, Optimasi Natrium Sitrat
dan Asam Fumarat sebagai Sumber Asam dalam
Pembuatan Granul Effervescent Ekstrak Temulawak
(Curcuma xanthorrhiza Roxb) secara Granulasi
Basah., Majalah Farmasi Indonesia, 18(1), 21-28.
Depkes RI, 2000, Parameter Standar Umum Ekstrak
Tumbuhan Obat. Departemen Kesehatan Republik
Indonesia, Jakarta.
Dwijayanti, R., 2009, Pemanfaatan Natrium Alginat
sebagai Fortifikasi Serat dalam Pembuatan Minuman
Serbuk Effervescent Bercitarasa Jeruk Lemon, Skripsi,
Fakultas Perikanan dan Ilmu Kelautan Institusi
Pertanian Bogor.
Formulation of Sechium edule Extract Effervescent Granule with the Variation of Citric Acid, Tartrate Acid and Sodium Bicarbonate
59

Fausett, H, Gayser C. and Dash AK., 2000, Evaluation of
Quick Disintegrating Calcium Carbonate Tablets. in:
Jurnal AAPS PharmScitech.
http://www.pharmscitech.com. accessed date 27
Agustus 2016.
Firdous, S.M., Sravanthi, K., Debnath, R., dan Neeraja,
K., 2012, Protective Effect of Ethanolic Extract and its
Ethylacetate and N-butanol Fractions of Sechium
edule Fruits Againts Carbon Tetrachloride Induced
Hepatic Injury in Rats, Int J Pharm Sci, 4(1): 354-359.
Gandhu, S., Syed, F.H., Gone, S.K., Bodavula, S.S.R.,
2012., Anti-Ulcer Activity of Sechium edule Ethanolic
Fruit Extract., The Pharma Innovation Journal., ISSN
2277-7695., Vol. 1 No. 5.
Hagerman, A.E., 2002, Tannin Chemistry.
http://www.users.muohio.edu/hagermae/tannin.pdf (20
September 2015).
Hayu, T.S., 2015, Formulasi Tablet Effervescent
Antioksidan Ekstrak Kulit Manggis (Garcinia
mangostana L.) dengan Kombinasi Asam Sitrat-Asam
Tartrat, Naskah Publikasi, Fakultas Farmasi
Universitas Muhammadiyah Surakarta, Surakarta.
Itsagusman, 2013, Pengujian Organoleptik, Modul
Penanganan Mutu Fisis (Organoleptik), Program Studi
Teknologi Pangan Universitas Muhammadiyah
Semarang.
Kamble, M.B., Dumbre, R.K., Rangari, V.D., 2008,
Hepatoprotective Activity Studies of Herbal
Formulations. Int J Green Pharm, 2:147-51.
Lindberg, N., Engfors, H., Ericsson, T., 1992, Encylopedia
of Pharmaceutical Technology, Effervescent
Pharmaceutical in Swarbricck, J., Boylan, J.C., Vol 5,
45-71, Marcel Dekker, Inc., New York.
Mandagi, R., Gregoria, S.S.D., Erny, N., Lucia, M., 2015,
Formulasi Granul Effervescent Sari Buah Pala
(Myristica fragrans H.), Cocos E-Journal, Vol. 6
No.2.
Mangunwardoyo, W., Ismaini, L., and Endang, S.H.,
2008., Analisis Senyawa Bioaktif dari Ekstrak Biji
Picung (Pangium edule Reinw)., Segar., Berita
Biologi, 9(3).
Marliana, S.D., Suryanti, V., Suyono., 2005, Skrining
Fitokimia dan analisis kromatografi lapis tipis
komponen kimia buah labu siam (Sechium edule Jacq.
Swartz.) dalam ekstrak etanol, Biofarmasi, 3(1):26-
31.
Mojab, F., Kamalinejad, M., Ghaderi, N., dan Vahidipour,
H.R., 2003, Phytochemical Screening of Some Species
of Iranian Plants, Iranian Journal of Pharmaceutical
Research, pp. 77-82.
Mohrle, R., 1980, Effervescent Tablet, in Lieberman,
H.A., Lachman, L., (eds),
Pharmaceutical Dosage
Form, Tablet, Vol. I, 284-362, Warner Lambert
Company, Morris Pliains, New Jersey.
Nugroho, S., 1999, Penambahan komponen berprotein
pada minuman serbuk effervescent, Skripsi, Fakultas
Teknologi Pertanian, IPB, Bogor.
Parikh, D.M., 2005, Handbook of Pharmaceutical
Granulation Technology. Taylor & Francis Group.
London, pp. 212-216.
Pratiwi, S.I.R., 2011, Karakterisasi Simplisia dan Uji
Aktivitas Antioksidan FEkstrak n-Heksan, Etil Asetat,
dan Etanol Herba Labu Siam (Sechium edule
(Jacq)Sw.) dengan metode DPPH. Skripsi. Fakultas
Farmasi Universitas Sumatra Utara, Medan.
Pulungan, H.M., Suprayogi, dan Yudha, B., 2004,
Effervescent Tanaman Obat. Trubus Agrisarana,
Surabaya.
Rizal, D., dan Widya, D.R.P., 2014, Pembuatan serbuk
effervescent miana (Coleus (L) benth): Kajian
konsentrasi dekstrin asam sitrat terhadap karakteristik
serbuk effervescent, Jurnal Pangan dan Agroindustri,
Vol.2 No.4.
Robinson, T., 1995, Kandungan Organik Tumbuhan
Tinggi, translated by Padmawinata K, 191-213.
Rofifah, D.S., 2016, Aktivitas Anti-Ulser Ekstrak Etanol
Buah Labu Siam (Sechium edule) Terhadap Ulkus
Peptikum Lambung Tikus Wistar, Skripsi, Fakultas
Farmasi Universitas Ahmad Dahlan.
Singh-Ackbarali, D., dan Maharaj, R., 2014, Sensory
Evaluation as a Tool in Determining Acceptability of
Innovative Products Developed by Undergraduate
Students in Food Science and Technology at The
University of Trinidad and Tobago, Journal of
Curriculum and Teaching, 3 (1), 10-27.
Siregar CJP., 2007, Teknologi Farmasi Sediaan Tablet
Dasar-Dasar Praktis. Penerbit EGC. Bandung. Hlm.
275, 277-280.
Sri, R.I., Silvia, R.Y., 2014, Pengaruh Perbandingan
Pelarut Etanol-Air Terhadap Kadar Tanin pada
Sokletasi Daun Gambir (Uncaria gambir Roxb), Sagu
Vol.13 No.1 : 1-7.
Sulistyani, N., dan Eka, K., 2011, Aktivitas Antifungi
Ekstrak Etanol Batang Binahong (Anredera cordifolia
(Tenore) Steen.) terhadap Candida albicans serta
Skrining Fitokimia, Jurnal Ilmiah Kefarmasian,
Vol.1,No.2,51-62.
Voigt, R., 1984, Buku Pelajaran Teknologi Farmasi,
translated by Soendani Noeromo, Universitas
Indonesia, Jakarta.
Voigt, R., 1995, Buku Pelajaran Teknologi Farmasi,
translated by Soendani N.S., UGM Press, Yogyakarta.
Widayanti, A., Naniek, S.R., and Dwi, O., 2012,
Optimasi Konsentrasi Asam Sitrat dan Asam Tartrat
(1: 2) sebagai Sumber Asam Ditinjau dari Sifat Fisik
Granul Effervescent Sari Buah Mengkudu (Morinda
citrifolia L), Farmasains,
Vol.1 No.6.
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
60
